Abstract
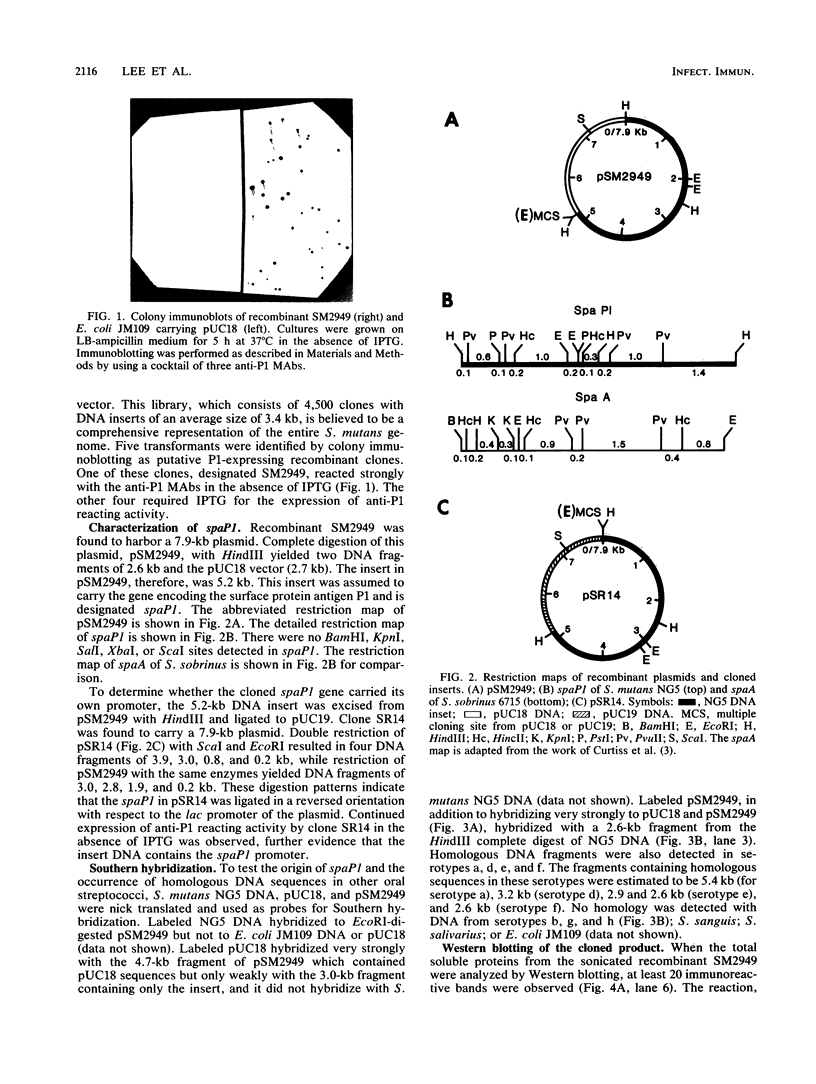
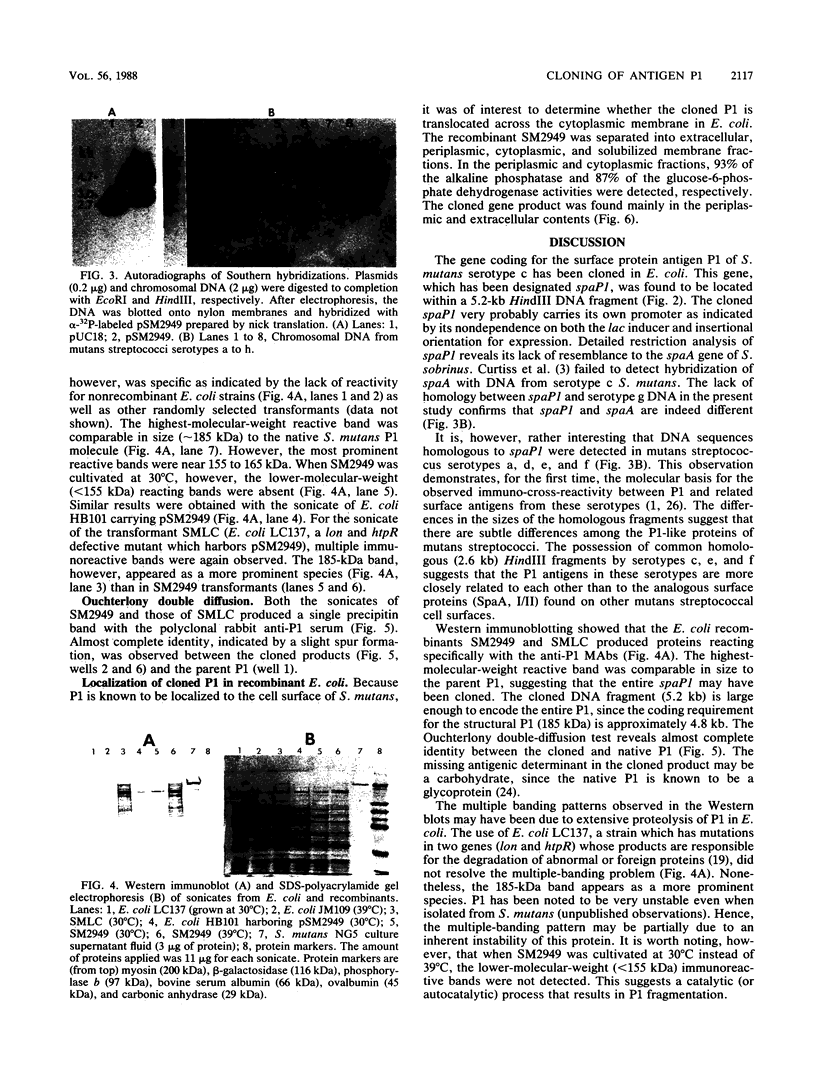
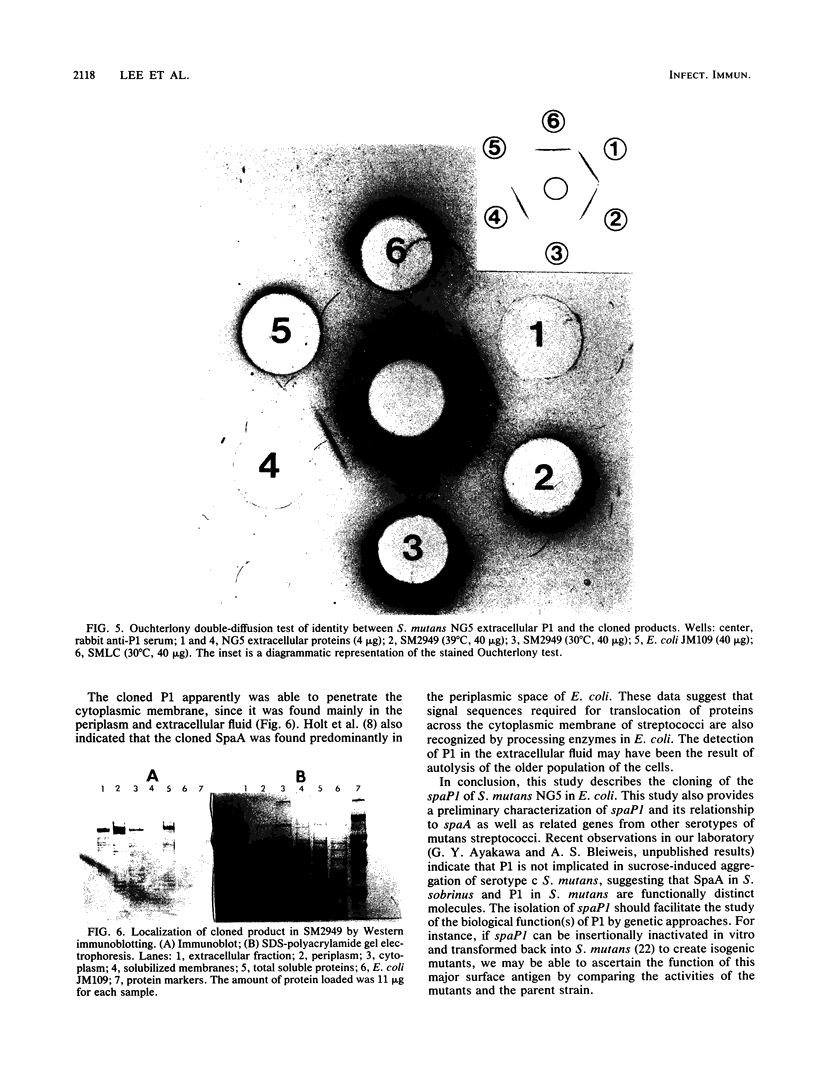
Antigen P1, also called I/II, is one of the most abundant cell wall proteins of the mutans streptococci. It has been suggested that P1 may be involved in cell adherence to tooth surfaces and in sucrose-induced cell aggregation. As a first step toward fully understanding its biological functions, the P1 gene, which has been designated spaP1, from Streptococcus mutans NG5 (serotype c) has been cloned into Escherichia coli JM109 by a shotgun procedure with pUC18 as the vector. The recombinant strain expressing P1 carries a 5.2-kilobase DNA insert whose restriction map has been determined. This map is completely different from that of spaA of Streptococcus sobrinus (serotype g), even though P1 and SpaA are antigenically related. Southern hybridization revealed that DNA sequences closely homologous to spaP1 were present in serotypes c, e, and f, and similar sequences also existed in strains of serotypes a and d. The expression of the cloned spaP1 was found to be independent of the lac inducer and the orientation of the DNA insert, suggesting that it carries its own promoter. Western blotting (immunoblotting) revealed at least 20 bands reacting with a mixture of three anti-P1 monoclonal antibodies. The highest-molecular-weight reactive band was comparable in size to the parent P1 (185 kilodaltons [kDa]); however, the major reactive bands were smaller (approximately 160 kDa). Expression of cloned P1 in E. coli LC137 (htpR lonR9) resulted in the increased prominence of the 185-kDa protein reactive band. Ouchterlony immunodiffusion showed partial identity between the parent and cloned P1. In E. coli, P1 was detected primarily in the periplasm and extracellular fluid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayakawa G. Y., Boushell L. W., Crowley P. J., Erdos G. W., McArthur W. P., Bleiweis A. S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J. T., Rappuoli R., Collier R. J. Expression of the S-1 catalytic subunit of pertussis toxin in Escherichia coli. Infect Immun. 1987 May;55(5):1321–1323. doi: 10.1128/iai.55.5.1321-1323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Beighton D., Cohen B. Immunisation of monkeys (Macaca fascicularis) with antigens purified from Streptococcus mutans. Br Dent J. 1982 Feb 2;152(3):81–84. doi: 10.1038/sj.bdj.4804751. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Peach S. L., Colman G., Cohen B. Antibody responses to antigens of Streptococcus mutans in monkeys (Macaca fascicularis) immunized against dental caries. J Gen Microbiol. 1983 Mar;129(3):865–875. doi: 10.1099/00221287-129-3-865. [DOI] [PubMed] [Google Scholar]
- Smith R., Lehner T., Beverley P. C. Characterization of monoclonal antibodies to Streptococcus mutans antigenic determinants I/II, I, II, and III and their serotype specificities. Infect Immun. 1984 Oct;46(1):168–175. doi: 10.1128/iai.46.1.168-175.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]







