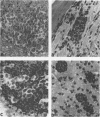
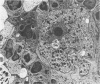
Abstract
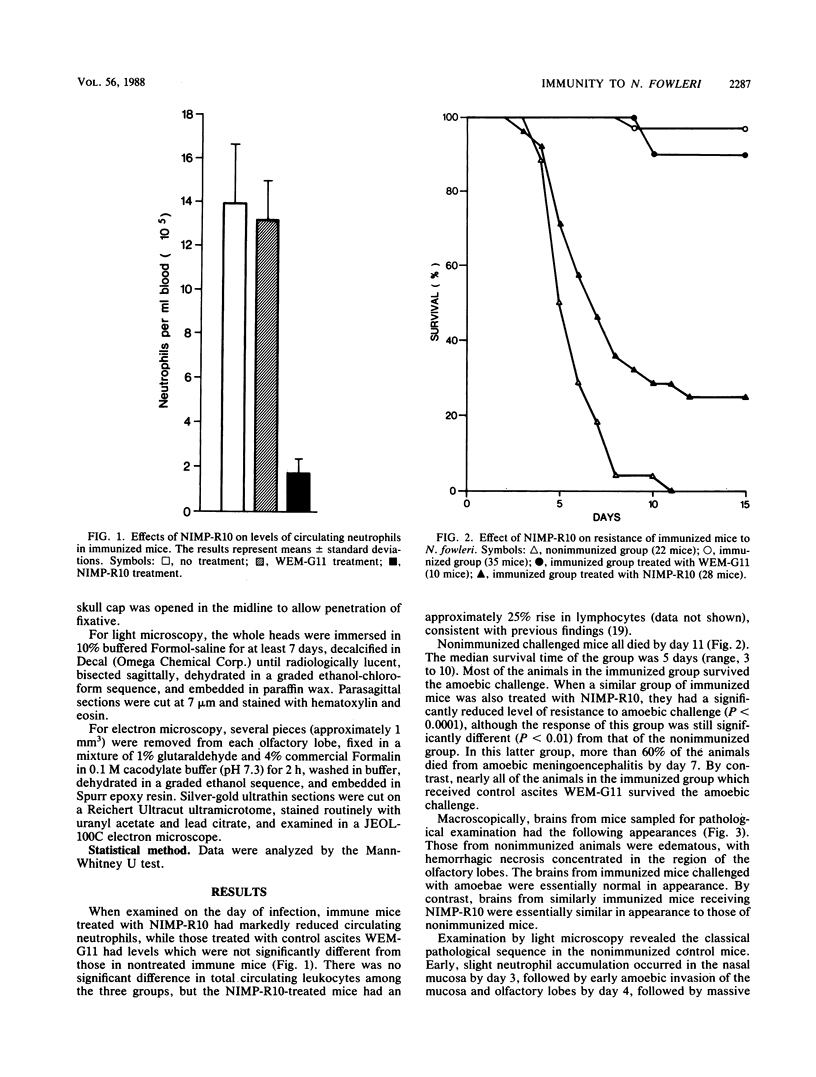
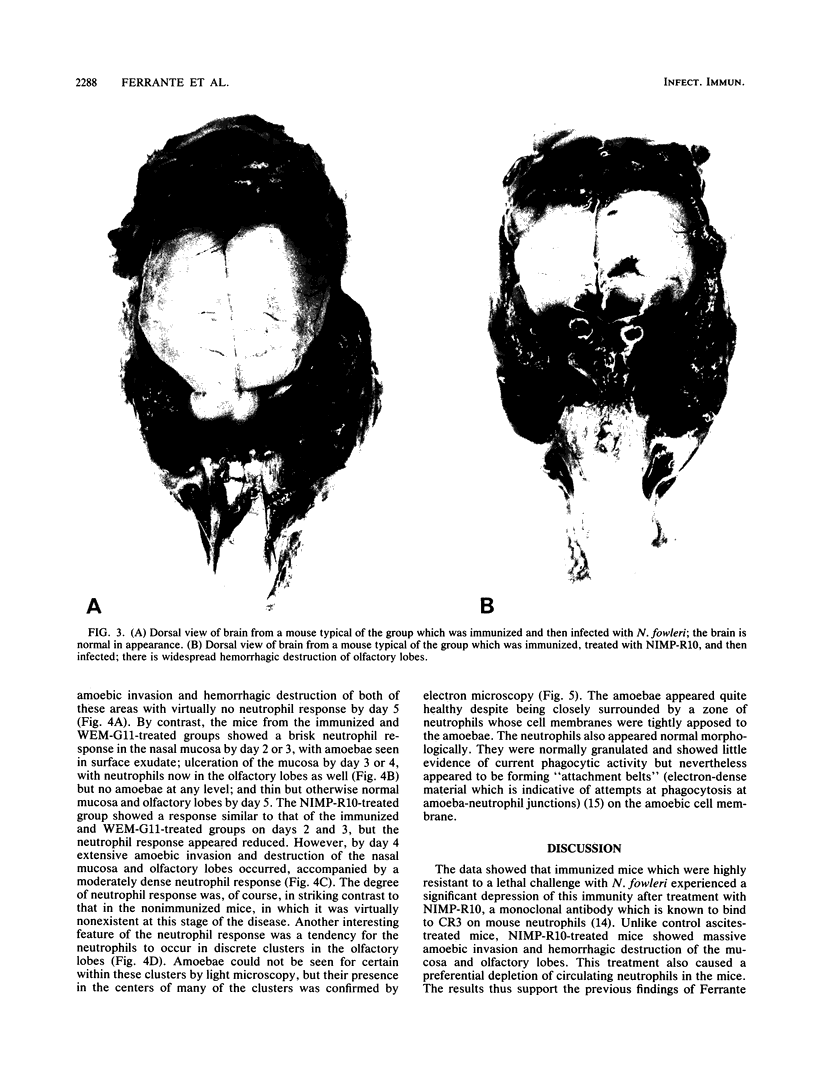
In an attempt to define the role of neutrophils in immunity to Naegleria fowleri in vivo, we examined the effects of treating immunized (with amoeba culture supernatant antigen) mice with the monoclonal antibody NIMP-R10, which binds to neutrophil complement receptor type 3bi (CR3) and causes selective neutrophil depletion in mice. Mice in the nonimmunized group challenged with amoebae all died by day 12, while 97% in the immunized group survived. By contrast, the immunized group treated with NIMP-R10 showed only 25% survival. The immunized group treated with "control" mouse ascites, WEM-G11, was highly resistant (90% survival). There was a significant neutrophil response in the nasal mucosa and olfactory lobes of immunized, NIMP-R10-treated mice, despite a marked degree of neutropenia similar to that seen in immunized, untreated mice. Nonimmunized mice showed virtually no neutrophil response. Despite this response in the NIMP-R10-treated mice, amoebic proliferation was not depressed, and there was no evidence of neutrophil degranulation or amoebic killing, despite the close apposition of large numbers of neutrophils to amoebae. The results indicate that neutrophils are necessary for the expression of immunity to N. fowleri.
Full text
PDF
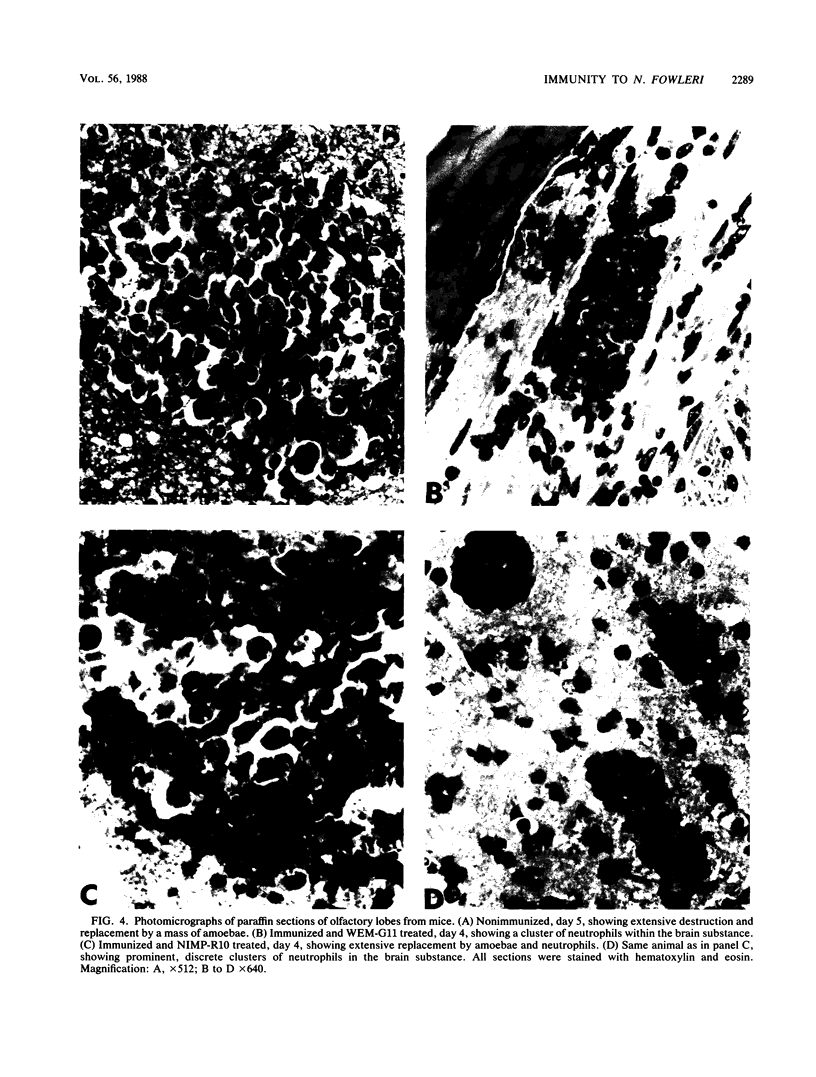
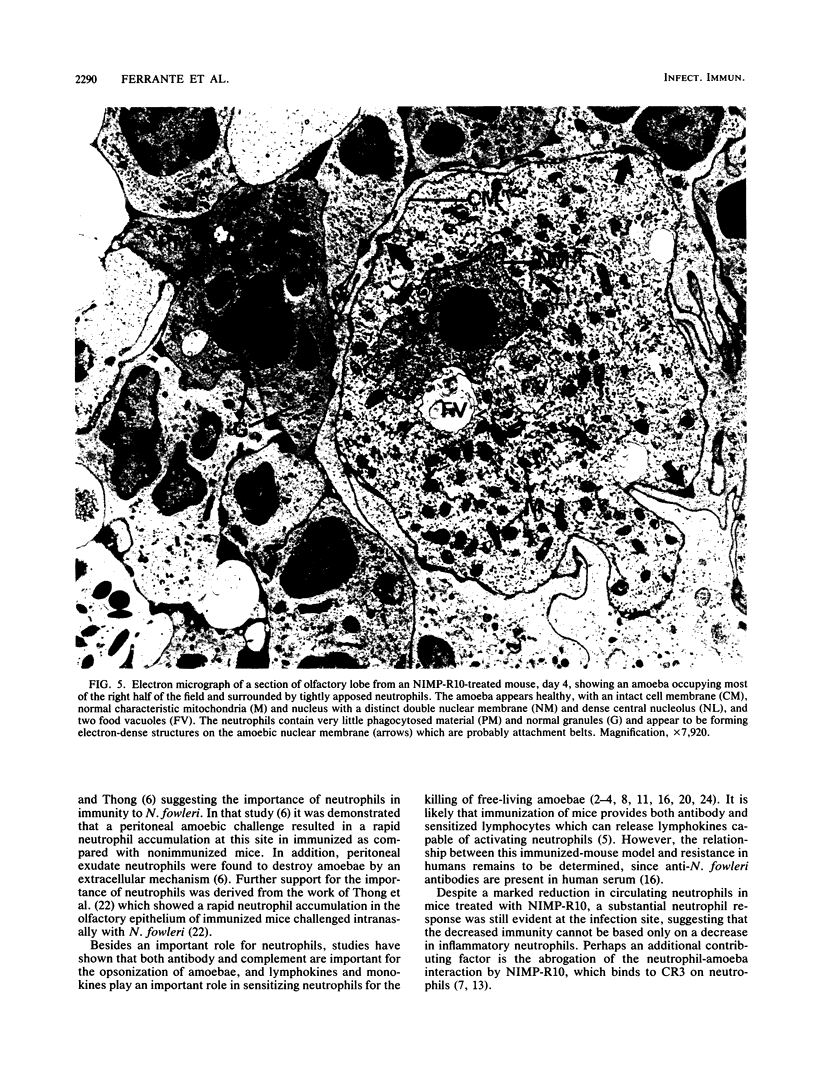

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter R. F. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg. 1972;66(2):193–213. doi: 10.1016/0035-9203(72)90147-2. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Abell T. J. Conditioned medium from stimulated mononuclear leukocytes augments human neutrophil-mediated killing of a virulent Acanthamoeba sp. Infect Immun. 1986 Feb;51(2):607–617. doi: 10.1128/iai.51.2.607-617.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Hill N. L., Abell T. J., Pruul H. Role of myeloperoxidase in the killing of Naegleria fowleri by lymphokine-altered human neutrophils. Infect Immun. 1987 May;55(5):1047–1050. doi: 10.1128/iai.55.5.1047-1050.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Mocatta T. J. Human neutrophils require activation by mononuclear leucocyte conditioned medium to kill the pathogenic free-living amoeba, Naegleria fowleri. Clin Exp Immunol. 1984 Jun;56(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Nandoskar M., Bates E. J., Goh D. H., Beard L. J. Tumour necrosis factor beta (lymphotoxin) inhibits locomotion and stimulates the respiratory burst and degranulation of neutrophils. Immunology. 1988 Mar;63(3):507–512. [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nikoloutsopoulos A., Lopez A. F., Vadas M. A., McDonald P. J., Finlay-Jones J. J. Role of cell surface receptors in the regulation of intracellular killing of bacteria by murine peritoneal exudate neutrophils. Infect Immun. 1986 Apr;52(1):245–251. doi: 10.1128/iai.52.1.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook T. W., Boackle R. J., Parker B. W., Vesely J. Activation of the alternative complement pathway by Naegleria fowleri. Infect Immun. 1980 Oct;30(1):58–61. doi: 10.1128/iai.30.1.58-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John D. T. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu Rev Microbiol. 1982;36:101–123. doi: 10.1146/annurev.mi.36.100182.000533. [DOI] [PubMed] [Google Scholar]
- John D. T., Weik R. R., Adams A. C. Immunization of mice against Naegleria fowleri infection. Infect Immun. 1977 Jun;16(3):817–820. doi: 10.1128/iai.16.3.817-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallinger G. J., Reiner S. L., Cooke D. W., Toffaletti D. L., Perfect J. R., Granger D. L., Durack D. T. Efficacy of immune therapy in early experimental Naegleria fowleri meningitis. Infect Immun. 1987 May;55(5):1289–1293. doi: 10.1128/iai.55.5.1289-1293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López A. F., Begley G., Andrews P., Butterworth A. E., Vadas M. A. Identification of a human granulocyte functional antigen (GFA-2) involved in antibody-dependent cell-mediated cytotoxicity and phagocytosis. J Immunol. 1985 Jun;134(6):3969–3977. [PubMed] [Google Scholar]
- López A. F., Burns G. F., Stanley I. J. Epitope diversity of monoclonal antibodies revealed by cross-species reactivity. Mol Immunol. 1984 May;21(5):371–374. doi: 10.1016/0161-5890(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Duma R. J., Nelson E. C., Moretta F. L. Experimental naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by Naegleria and pathologic changes produced: a light and electron microscope study. Lab Invest. 1973 Aug;29(2):121–133. [PubMed] [Google Scholar]
- Pentilla I. A., Ey P. L., Lopez A. F., Jenkin C. R. Suppression of early immunity to Nematospiroides dubius in mice by selective depletion of neutrophils with monoclonal antibody. Aust J Exp Biol Med Sci. 1985 Oct;63(Pt 5):531–543. doi: 10.1038/icb.1985.57. [DOI] [PubMed] [Google Scholar]
- Rowan-Kelly B., Ferrante A., Thong Y. H. Activation of complement by Naegleria. Trans R Soc Trop Med Hyg. 1980;74(3):333–336. doi: 10.1016/0035-9203(80)90092-9. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Carter R. F., Ferrante A., Rowan-Kelly B. Site of expression of immunity to Naegleria fowleri in immunized mice. Parasite Immunol. 1983 Jan;5(1):67–76. doi: 10.1111/j.1365-3024.1983.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B., O'Keefe D. Immunization with live amoebae, amoebic lysate and culture supernatant in experimental Naegleria meningoencephalitis. Trans R Soc Trop Med Hyg. 1980;74(5):570–576. doi: 10.1016/0035-9203(80)90141-8. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Shepherd C., Rowan-Kelly B. Resistance of mice to Naegleria meningoencephalitis transferred by immune serum. Trans R Soc Trop Med Hyg. 1978;72(6):650–652. doi: 10.1016/0035-9203(78)90025-1. [DOI] [PubMed] [Google Scholar]
- Whiteman L. Y., Marciano-Cabral F. Susceptibility of pathogenic and nonpathogenic Naegleria spp. to complement-mediated lysis. Infect Immun. 1987 Oct;55(10):2442–2447. doi: 10.1128/iai.55.10.2442-2447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]