Abstract
This review summarizes heme metabolism and focuses especially upon the control of hepatic heme biosynthesis. Activity of δ-aminolevulinic acid synthetase, the first enzyme of heme biosynthesis, is of primary importance in controlling the overall activity of this biosynthetic pathway. Δ-aminolevulinic acid synthetase is subject to inhibition and repression by heme, and numerous basic and clinical studies support the concept that there exists within hepatocytes a “regulatory” heme pool which controls activity of δ-aminolevulinic acid synthetase. In addition, activity of this enzyme is repressed by feeding, especially by ingestion of carbohydrates (the so-called “glucose effect”). Studies pertaining to the mechanisms underlying this effect are also reviewed. The “glucose effect” appears to be mediated by glucose or perhaps by glucose-6-phosphate or uridine diphosphate glucose, rather than by metabolites further removed from glucose itself. Unlike the situation in E. coli, the “glucose effect” in liver of higher organisms is not mediated by alterations in intracellular concentrations of cyclic AMP. Effects of heavy metals, especially iron, on hepatic heme metabolism are also considered. Iron has been found to inhibit formation and utilization of uroporphyrinogen III and to lead to decreased concentrations of microsomal heme and cytochrome P-450. Administration of large amounts of iron is also associated with an increase in activity of heme oxygenase, a property shared by several other metal ions, most notably cobalt. This effect of iron or cobalt administration is similar to the effect of heme administration in increasing heme oxygenase activity; however, we believe it is unlikely that iron, rather than heme itself, is a physiologic regulator of hepatic heme metabolism, although this hypothesis has lately been proposed.
Full text
PDF

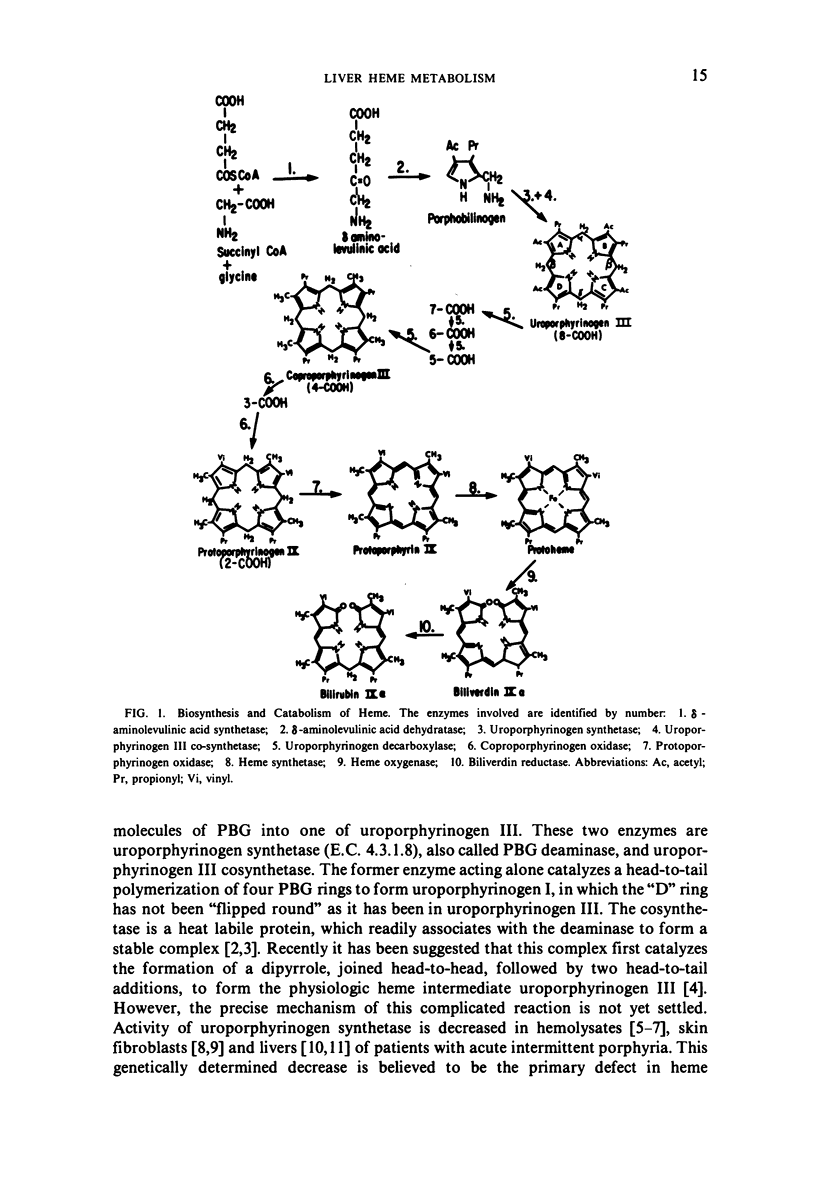


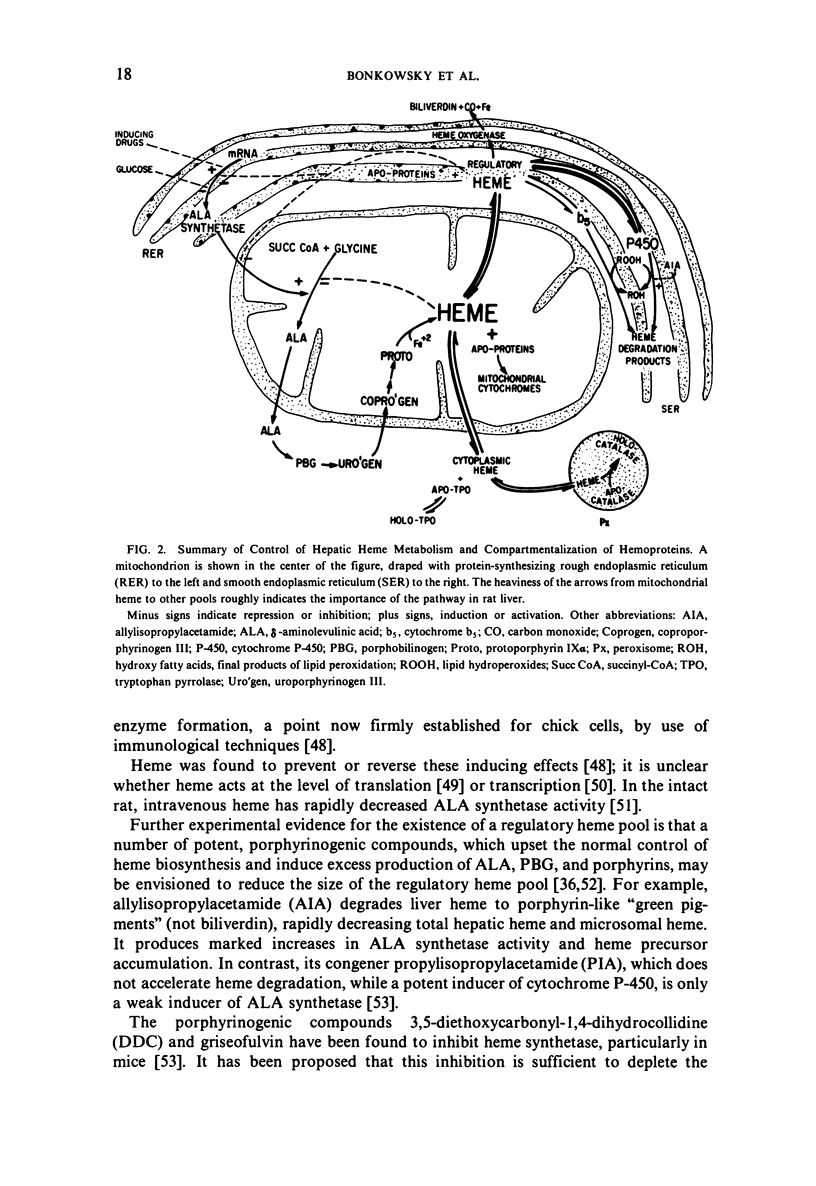

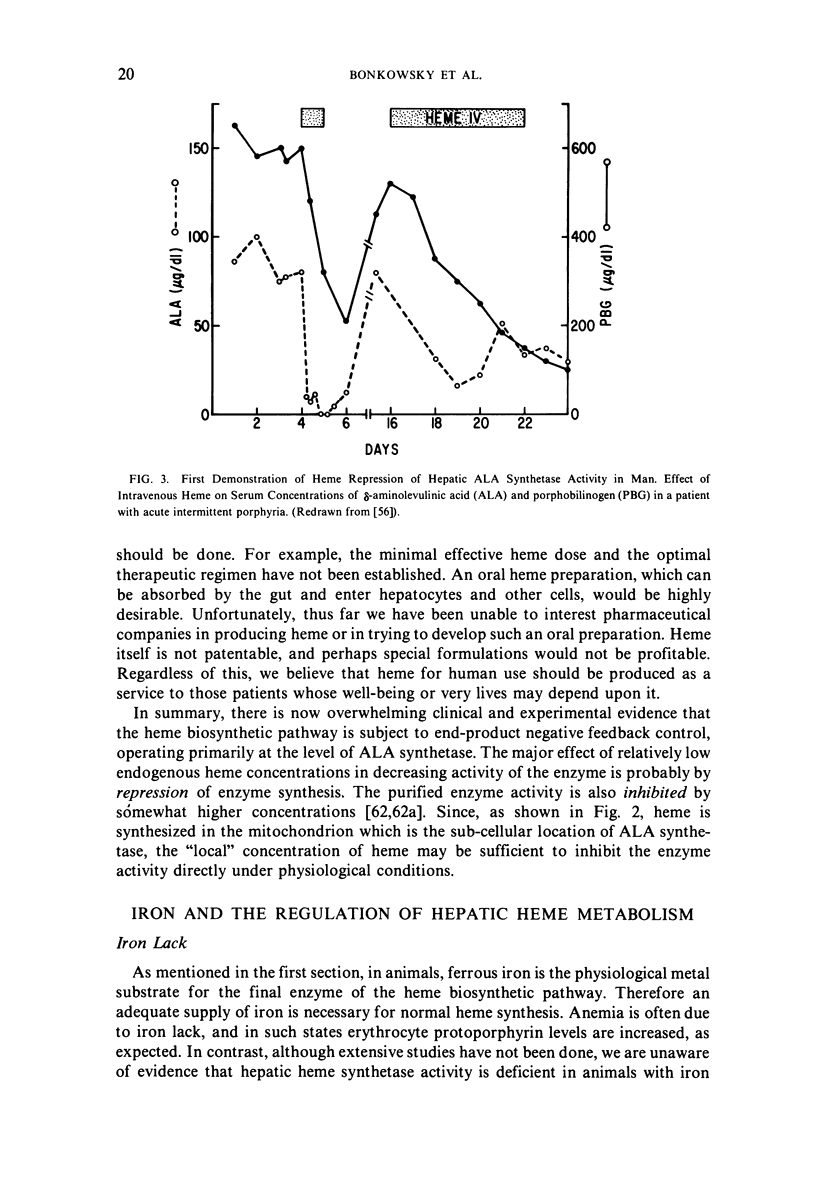

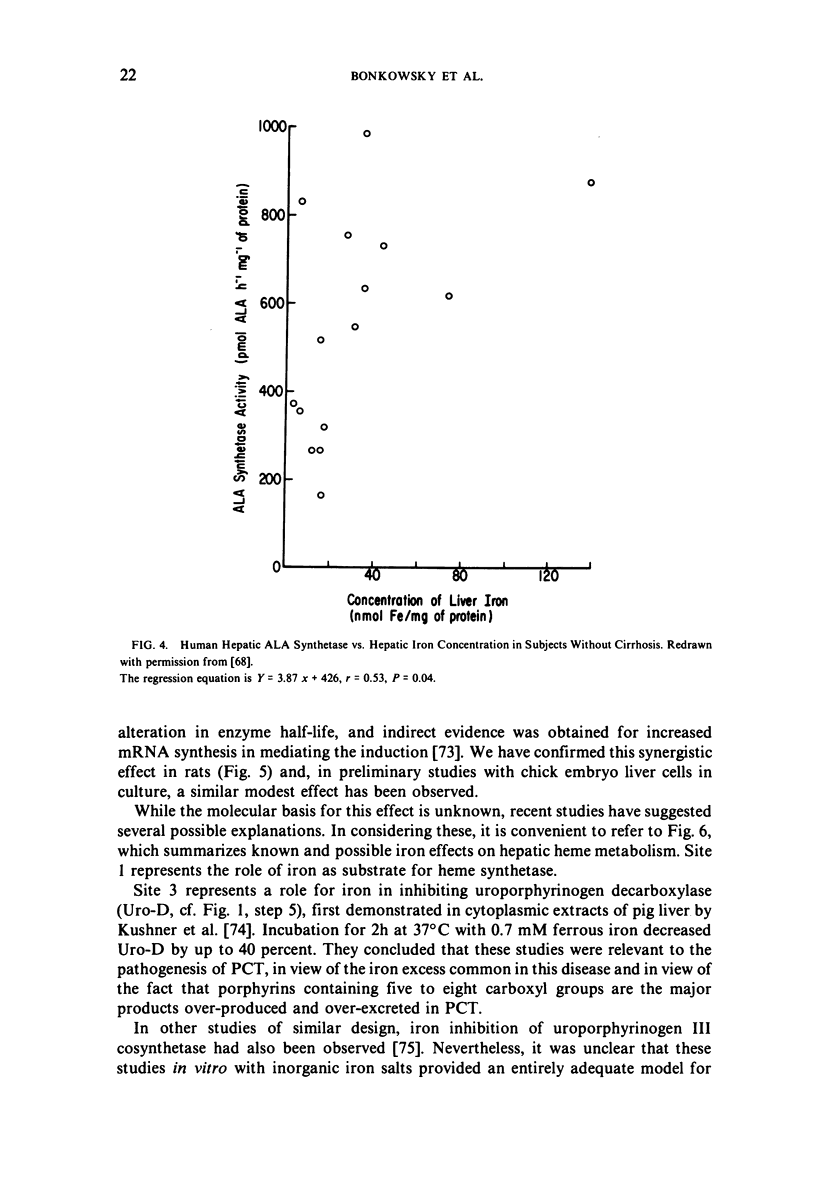
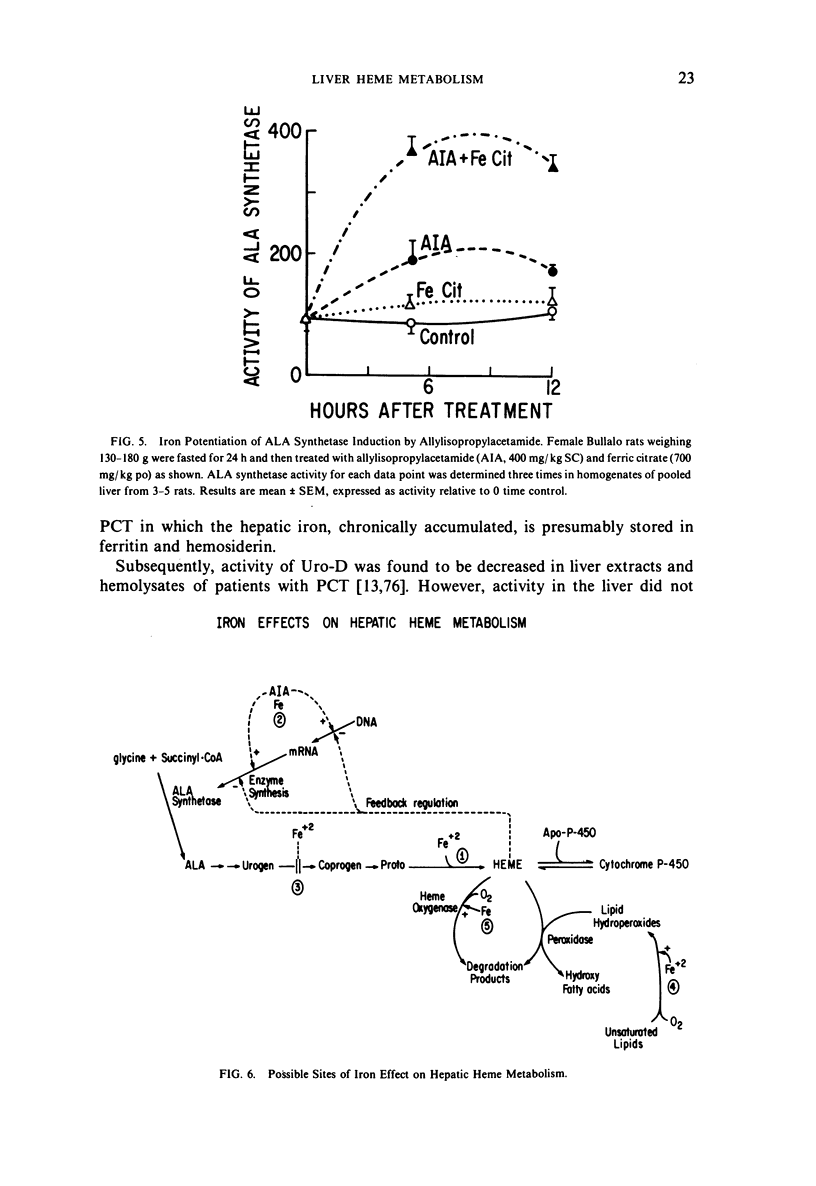

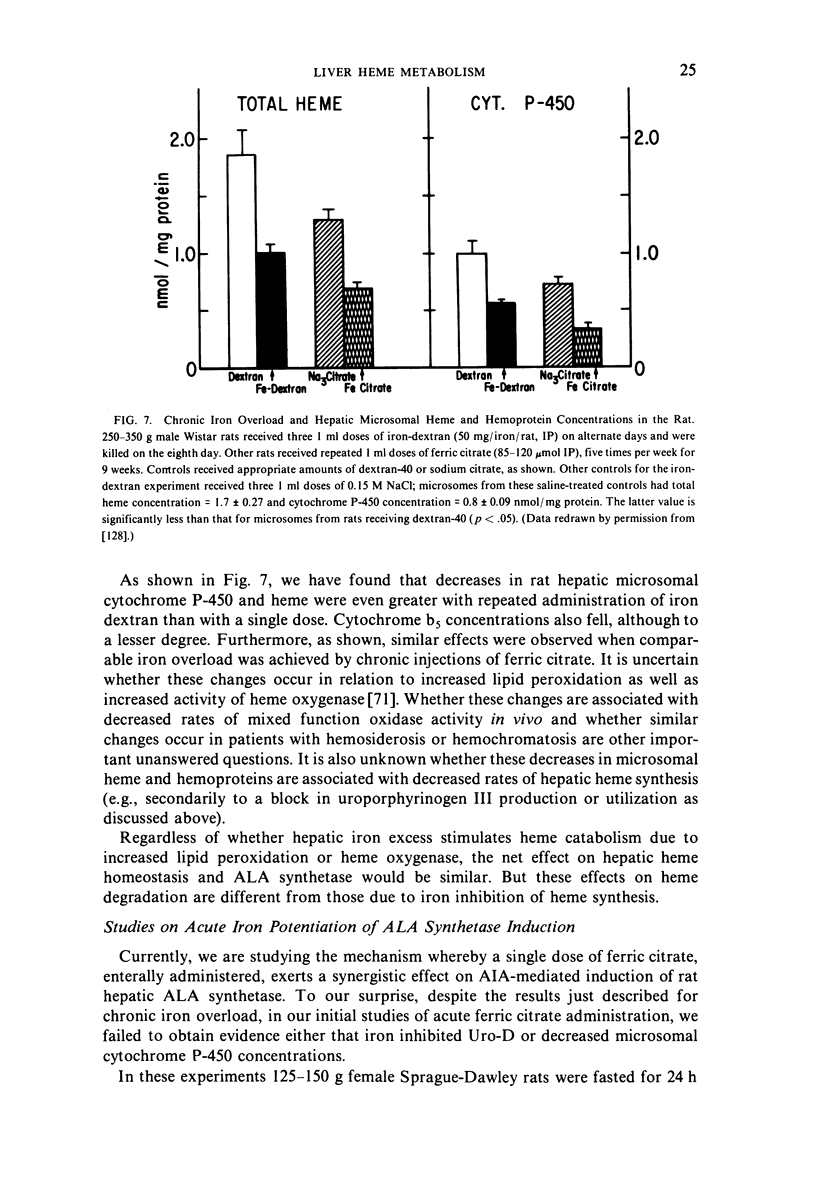
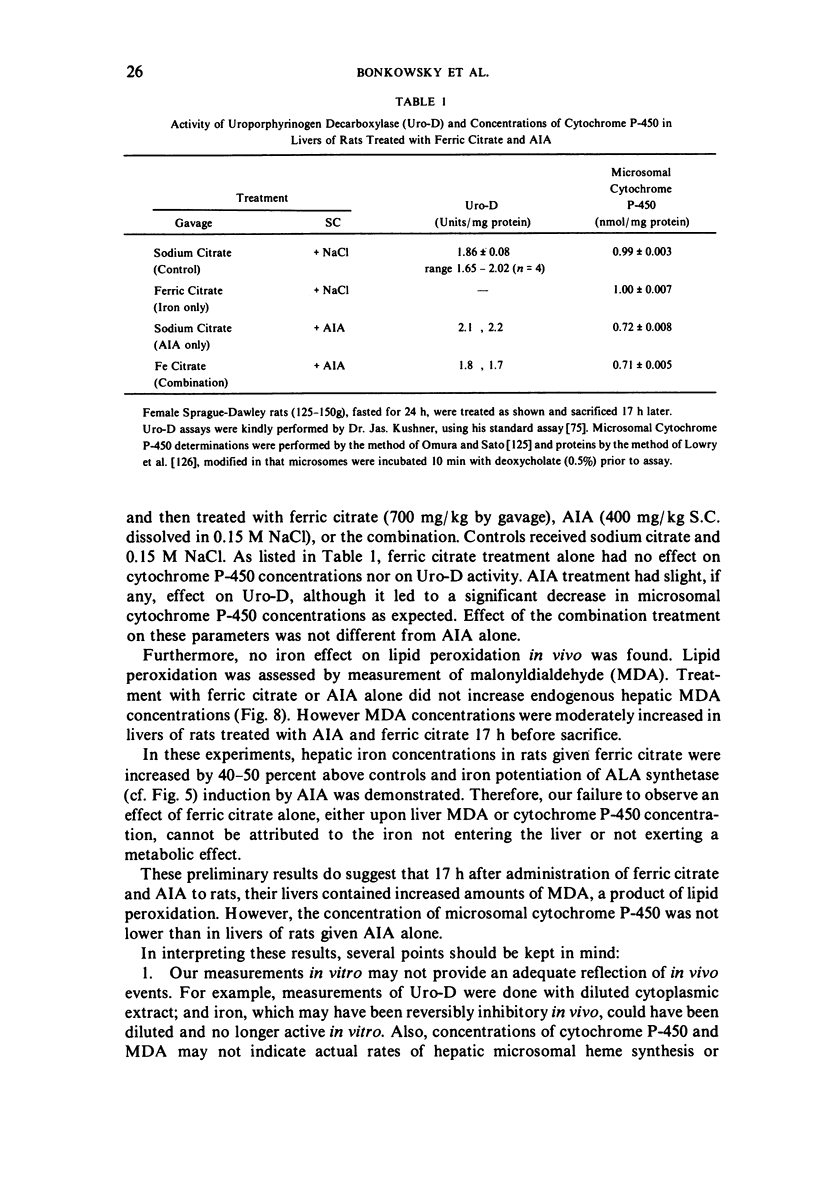
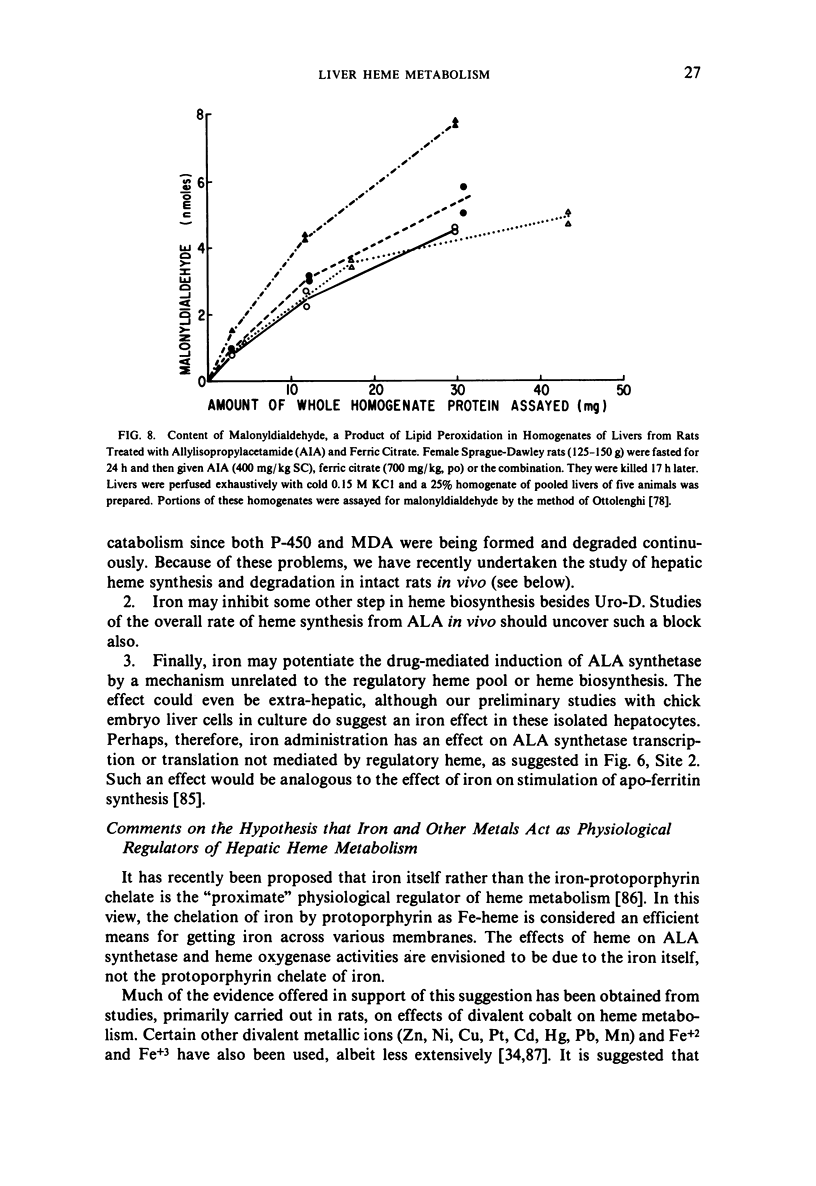









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E. Effects of antihypertensive drugs on hepatic heme biosynthesis, and evaluation of ferrochelatase inhibitors to simplify testing of drugs for heme pathway induction. Biochim Biophys Acta. 1978 Oct 18;543(3):313–327. doi: 10.1016/0304-4165(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Bailey-Wood R., Blayney L. M., Muir J. R., Jacobs A. The effects of iron deficiency on rat liver enzymes. Br J Exp Pathol. 1975 Jun;56(3):193–198. [PMC free article] [PubMed] [Google Scholar]
- Benedetto A. V., Kushner J. P., Taylor J. S. Porphyria cutanea tarda in three generations of a single family. N Engl J Med. 1978 Feb 16;298(7):358–362. doi: 10.1056/NEJM197802162980702. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E. Cytochrome p-450 heme and the regulation of delta-aminolevulinic acid synthetase in the liver. Arch Biochem Biophys. 1976 Sep;176(1):103–112. doi: 10.1016/0003-9861(76)90145-4. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Carpenter S. J., Healey J. F. Iron and the liver: subcellular distribution of iron and decreased microsomal cytochrome P-450 in livers of iron-loaded rats. Arch Pathol Lab Med. 1979 Jan;103(1):21–29. [PubMed] [Google Scholar]
- Bonkowsky H. L., Collins A., Doherty J. M., Tschudy D. P. The glucose effect in rat liver: studies of delta-aminolevulinate synthase and tyrosine aminotransferase. Biochim Biophys Acta. 1973 Oct 5;320(3):561–575. doi: 10.1016/0304-4165(73)90136-0. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Magnussen C. R., Collins A. R., Doherty J. M., Hess R. A., Tschudy D. P. Comparative effects of glycerol and dextrose on porphyrin precursor excretion in acute intermittent porphyria. Metabolism. 1976 Apr;25(4):405–414. doi: 10.1016/0026-0495(76)90072-x. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Pomeroy J. S. Human hepatic delta-aminolaevulinate synthase: requirement of an exogenous system for succinyl-coenzyme A generation to demonstrate increased activity in cirrhotic and anticonvulsant-treated subjects. Clin Sci Mol Med. 1977 May;52(5):509–521. doi: 10.1042/cs0520509. [DOI] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Collins A., Doherty J., Bossenmaier I., Cardinal R., Watson C. J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2725–2729. doi: 10.1073/pnas.68.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Weinbach E. C., Ebert P. S., Doherty J. M. Porphyrin synthesis and mitochondrial respiration in acute intermittent porphyria: studies using cultured human fibroblasts. J Lab Clin Med. 1975 Jan;85(1):93–102. [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- Brodie M. J., Moore M. R., Thompson G. G., Goldberg A. The treatment of acute intermittent prophyria with laevulose. Clin Sci Mol Med. 1977 Oct;53(4):365–371. doi: 10.1042/cs0530365. [DOI] [PubMed] [Google Scholar]
- Brodie M. J., Thompson G. G., Moore M. R., Beattie A. D., Goldberg A. Hereditary coproporphyria. Demonstration of the abnormalities in haem biosynthesis in peripheral blood. Q J Med. 1977 Apr;46(182):229–241. [PubMed] [Google Scholar]
- Catz C. S., Juchau M. R., Yaffe S. J. Effects of iron, riboflavin and iodide deficiencies on hepatic drug-metabolizing enzyme systems. J Pharmacol Exp Ther. 1970 Aug;174(2):197–205. [PubMed] [Google Scholar]
- Conn H. O. Portacaval anastomosis and hepatic hemosiderin deposition: a prospective, controlled investigation. Gastroenterology. 1972 Jan;62(1):61–72. [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Ferrochelatase activity in wild-type and mutant strains of Spirillum itersonii. Solubilization with chaotropic reagents. Arch Biochem Biophys. 1974 Feb;160(2):523–529. doi: 10.1016/0003-9861(74)90429-9. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Inhibition of haem synthesis caused by cobalt in rat liver. Evidence for two different sites of action. Biochem J. 1977 Jan 15;162(1):213–216. doi: 10.1042/bj1620213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. The effect of cobaltous chloride on liver haem metabolism in the rat. Evidence for inhibition of haem synthesis and for increased haem degradation. Ann Clin Res. 1976;8 (Suppl 17):193–197. [PubMed] [Google Scholar]
- De Matteis F., Sparks R. G. Iron-dependent loss of liver cytochrome P-450 haem in vivo and in vitro. FEBS Lett. 1973 Jan 15;29(2):141–144. doi: 10.1016/0014-5793(73)80545-9. [DOI] [PubMed] [Google Scholar]
- Delta-Aminolevulinic acid synthase from chick embryo liver mitochondria. II. Immunochemical correlation between synthesis and activity in induction and repression. J Biol Chem. 1976 Mar 10;251(5):1347–1353. [PubMed] [Google Scholar]
- Dhar G. J., Bossenmaier I., Petryka Z. J., Cardinal R., Watson C. J. Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975 Jul;83(1):20–30. doi: 10.7326/0003-4819-83-1-20. [DOI] [PubMed] [Google Scholar]
- Doss M., Look D., Henning H., Nawrocki P., Schmidt A., Dölle W., Korb G., Lüders C. J., Strohmeyer G. Hepatic porphyrins and urinary porphyrins and porphyrin precursors in liver cirrhosis. Klin Wochenschr. 1972 Nov 15;50(22):1025–1032. doi: 10.1007/BF01486762. [DOI] [PubMed] [Google Scholar]
- Edwards A. M., Elliott W. H. Induction of delta-aminolevulinic acid synthetase in isolated rat liver cell suspensions. Adenosine 3':5'-monophosphate dependence of induction by drugs. J Biol Chem. 1974 Feb 10;249(3):851–855. [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Thomas N. The primary enzyme defect in hereditary coproporphyria. Lancet. 1976 Dec 4;2(7997):1217–1219. doi: 10.1016/s0140-6736(76)91143-0. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Lee G. B., Tovey J. A. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978 Aug 10;299(6):274–278. doi: 10.1056/NEJM197808102990603. [DOI] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. H., Redeker A. G. Porphyria cutanea tarda. A study of the effect of phlebotomy. N Engl J Med. 1968 Dec 12;279(24):1301–1304. doi: 10.1056/NEJM196812122792402. [DOI] [PubMed] [Google Scholar]
- Felsher B. F., Jones M. L., Redeker A. G. Iron and hepatic uroporphyrin synthesis. Relation in porphyria cutanea tarda. JAMA. 1973 Nov 5;226(6):663–665. [PubMed] [Google Scholar]
- Felsher B. F., Redeker A. G. Acute intermittent porphyria: effect of diet and griseofulvin. Medicine (Baltimore) 1967 Mar;46(2):217–223. doi: 10.1097/00005792-196703000-00014. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Feinstein G. Studies on porphobilinogen deaminase and uroporphyrinogen 3 cosynthase from human erythrocytes. Biochim Biophys Acta. 1974 Jun 18;350(2):358–373. doi: 10.1016/0005-2744(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Valasinas A., Frydman B. Mechanism of uroporphyrinogen biosynthesis from porphobilinogen. Enzyme. 1973;16(1):151–159. doi: 10.1159/000459375. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Goldberg M. L. The glucose effect: carbohydrate repression of enzyme induction, RNA synthesis, and glucocorticoid activity -- a role for cyclic AMP and cyclic GMP. Life Sci. 1975 Dec 15;17(12):1747–1754. doi: 10.1016/0024-3205(75)90456-7. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Nordmann Y. Decreased lymphocyte coproporphyrinogen III oxidase activity in hereditary coproporphyria. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1089–1095. doi: 10.1016/0006-291x(77)91630-8. [DOI] [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Guzelian P. S., Bissell D. M. Effect of cobalt on synthesis of heme and cytochrome P-450 in the liver. Studies of adult rat hepatocytes in primary monolayer culture and in vivo. J Biol Chem. 1976 Jul 25;251(14):4421–4427. [PubMed] [Google Scholar]
- HUNTER F. E., Jr, GEBICKI J. M., HOFFSTEN P. E., WEINSTEIN J., SCOTT A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem. 1963 Feb;238:828–835. [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., O'Brien P. J. Cytochrome P-450 as a microsomal peroxidase utilizing a lipid peroxide substrate. Arch Biochem Biophys. 1971 Nov;147(1):14–27. doi: 10.1016/0003-9861(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Igarashi J., Hayashi N., Kikuchi G. Effects of administration of cobalt chloride and cobalt protoporphyrin on delta-aminolevulinate synthase in rat liver. J Biochem. 1978 Oct;84(4):997–1000. doi: 10.1093/oxfordjournals.jbchem.a132215. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Games D. E., Couch P., Jackson J. R., Belcher R. B., Smith S. G. Conversion of coproporphyrinogen 3 to protoporphyrin IX. Enzyme. 1974;17(1):81–87. doi: 10.1159/000459311. [DOI] [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M. Nitrate, fumarate, and oxygen as electron acceptors for a late step in microbial heme synthesis. Biochim Biophys Acta. 1976 Oct 13;449(1):1–9. doi: 10.1016/0005-2728(76)90002-5. [DOI] [PubMed] [Google Scholar]
- Kassner R. J., Walchak H. Heme formation from Fe(II) and porphyrin in the absence of ferrochelatase activity. Biochim Biophys Acta. 1973 Apr 28;304(2):294–303. doi: 10.1016/0304-4165(73)90247-x. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kikuchi G. Possible participation of cyclic AMP in the regulation of -aminolevulinic acid synthesis in rat liver. J Biochem. 1972 May;71(5):923–926. doi: 10.1093/oxfordjournals.jbchem.a129847. [DOI] [PubMed] [Google Scholar]
- Korinek J., Moses H. L. Theophylline suppression of delta-aminolevulinic acid synthetase induction in chick embryo and rat livers. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1246–1252. doi: 10.1016/0006-291x(73)90599-8. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. P., Lee G. R., Nacht S. The role of iron in the pathogenesis of porphyria cutanea tarda. An in vitro model. J Clin Invest. 1972 Dec;51(12):3044–3051. doi: 10.1172/JCI107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. P., Steinmuller D. P., Lee G. R. The role of iron in the pathogenesis of porphyria cutanea tarda. II. Inhibition of uroporphyrinogen decarboxylase. J Clin Invest. 1975 Sep;56(3):661–667. doi: 10.1172/JCI108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Jacobson M., Kuntzman R., Poyer J. L., McCay P. B. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch Biochem Biophys. 1973 Oct;158(2):842–852. doi: 10.1016/0003-9861(73)90580-8. [DOI] [PubMed] [Google Scholar]
- Magnussen C. R., Levine J. B., Doherty J. M., Cheesman J. O., Tschudy D. P. A red cell enzyme method for the diagnosis of acute intermittent porphyria. Blood. 1974 Dec;44(6):857–868. [PubMed] [Google Scholar]
- Maines M. D., Janousek V., Tomio J. M., Kappas A. Cobalt inhibition of synthesis and induction of delta-aminolevulinate synthase in liver. Proc Natl Acad Sci U S A. 1976 May;73(5):1499–1503. doi: 10.1073/pnas.73.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Enzymes of heme metabolism in the kidney: regulation by trace metals which do not form heme complexes. J Exp Med. 1977 Nov 1;146(5):1286–1293. doi: 10.1084/jem.146.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Sinclair P. Cobalt regulation of heme synthesis and degradation in avian embryo liver cell culture. J Biol Chem. 1977 Jan 10;252(1):219–223. [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P., Rechcigl M., Jr Delta-aminolevulinic acid synthetase. II. Induction in rat liver. J Biol Chem. 1966 Oct 10;241(19):4323–4329. [PubMed] [Google Scholar]
- Marver H. S., Collins A., Tschudy D. P. The 'permissive' effect of hydrocortisone on the induction of delta-aminolaevulate synthetase. Biochem J. 1966 Jun;99(3):31C–33C. [PMC free article] [PubMed] [Google Scholar]
- Marver H. S., Tschudy D. P., Perlroth M. G., Collins A. Delta-aminolevulinic acid synthetase. I. Studies in liver homogenates. J Biol Chem. 1966 Jun 25;241(12):2803–2809. [PubMed] [Google Scholar]
- Maxwell J. D., Meyer U. A. Effect of lead on hepatic delta-aminolaevulinic acid synthetase activity in the rat: a model for drug sensitivity in intermittent acute porphyria. Eur J Clin Invest. 1976 Sep 10;6(5):373–379. doi: 10.1111/j.1365-2362.1976.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Miyagi K., Cardinal R., Bossenmaier I., Watson C. J. The serum porphobilinogen and hepatic porphobilinogen deaminase in normal and porphyric individuals. J Lab Clin Med. 1971 Nov;78(5):683–695. [PubMed] [Google Scholar]
- Morgan R. O., Stephens J. K., Fischer P. W., Marks G. S. Drug-induced porphyrin biosynthesis--XVI. Effects of hydrocortisone, adenosine 3':5'-cyclic monophosphoric acid, its dibutyryl derivative and a phosphodiesterase inhibitor on allylisopropylacetamide-induced porphyrin biosynthesis in chick embryo liver cells maintained in serum-free Waymouth medium. Biochem Pharmacol. 1977 Aug 1;26(15):1389–1394. doi: 10.1016/0006-2952(77)90362-8. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- Narisawa K., Kikuchi G. Effect of inhibitors of DNA synthesis on allylisopropylacetamide-induced increases of delta-aminolevulinic acid synthetase and other enzymes in rat liver. Biochim Biophys Acta. 1965 Jun 22;99(3):580–583. doi: 10.1016/s0926-6593(65)80221-1. [DOI] [PubMed] [Google Scholar]
- Narisawa K., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in rat-liver mitochondria. Biochim Biophys Acta. 1966 Sep;123(3):596–605. doi: 10.1016/0005-2787(66)90226-7. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- PERAINO C., PITOT H. C. STUDIES ON THE INDUCTION AND REPRESSION OF ENZYMES IN RAT LIVER. II. CARBOHYDRATE REPRESSION OF DIETARY AND HORMONAL INDUCTION OF THREONINE DEHYDRASE AND ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1964 Dec;239:4308–4313. [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C. Carbohydrate repression of enzyme induction in rat liver. J Biol Chem. 1963 May;238:1910–1912. [PubMed] [Google Scholar]
- PORRA R. J., FALK J. E. Protein-bound porphyrins associated with protoporphyrin biosynthesis. Biochem Biophys Res Commun. 1961 Jun 28;5:179–184. doi: 10.1016/0006-291x(61)90106-1. [DOI] [PubMed] [Google Scholar]
- Peraino C., Lamar C., Jr, Pitot H. C. Studies on the mechanism of carbohydrate repression in rat liver. Adv Enzyme Regul. 1966;4:199–217. doi: 10.1016/0065-2571(66)90015-x. [DOI] [PubMed] [Google Scholar]
- Perlroth M. G., Tschudy D. P., Ratner A., Spaur W., Redeker A. The effect of diet in variegate (South African genetic) porphyria. Metabolism. 1968 Jul;17(7):571–581. doi: 10.1016/0026-0495(68)90015-2. [DOI] [PubMed] [Google Scholar]
- Peterson A., Bossenmaier I., Cardinal R., Watson C. J. Hematin treatment of acute porphyria. Early remission of an almost fatal relapse. JAMA. 1976 Feb 2;235(5):520–522. [PubMed] [Google Scholar]
- Pinelli A., Capuano A. ALA synthetase induction. Inhibitory effect exerted by administration of dibutyryl adenosine-3',5'-cyclic monophosphate, theophylline and caffeine. Enzyme. 1973;16(1):203–210. [PubMed] [Google Scholar]
- Pinelli A., Colombo A., Fumagalli D., Tofanetti O. 5-Aminolevulinic acid synthetase induction: inhibitory effect exerted by administration of uridine diphosphate glucose. Biochem Pharmacol. 1976 Mar 15;25(6):623–624. doi: 10.1016/0006-2952(76)90234-3. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Lamola A. A., Poh-Fitzpatrick M. F., Seaman C., Harber L. C. Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. I. Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J Clin Invest. 1975 Dec;56(6):1519–1527. doi: 10.1172/JCI108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Polglase W. J. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX. Protoporphyrinogen oxidase activity in mitochondrial extracts of Saccharomyces cerevisiae. J Biol Chem. 1975 Feb 25;250(4):1269–1274. [PubMed] [Google Scholar]
- Poulson R. The enzymic conversion of protoporphyrinogen IX to protoporphyrin IX in mammalian mitochondria. J Biol Chem. 1976 Jun 25;251(12):3730–3733. [PubMed] [Google Scholar]
- ROSE J. A., HELLMAN E. S., TSCHUDY D. P. Effect of diet on induction of experimental porphyria. Metabolism. 1961 Jul;10:514–521. [PubMed] [Google Scholar]
- Redeker A. G., Sterling R. E. The "glucose effect" in erythropoietic protoporphyria. Arch Intern Med. 1968 May;121(5):446–448. [PubMed] [Google Scholar]
- Romeo G., Levin E. Y. Uroporphyrinogen 3 cosynthetase in human congenital erythropoietic porphyria. Proc Natl Acad Sci U S A. 1969 Jul;63(3):856–863. doi: 10.1073/pnas.63.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Sancovich H. A., Battle A. M., Grinstein M. Porphyrin biosynthesis. VI. Separation and purification of porphobilinogen deaminase and uroporphyrinogen isomerase from cow liver. Porphobilinogenase an allosteric enzyme. Biochim Biophys Acta. 1969 Sep 30;191(1):130–143. doi: 10.1016/0005-2744(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B. A., Meyer U. A., Marver H. S. Hemoprotein catabolism during stimulation of microsomal lipid peroxidation. Biochim Biophys Acta. 1972 Aug 18;279(1):221–227. doi: 10.1016/0304-4165(72)90259-0. [DOI] [PubMed] [Google Scholar]
- Scholnick P. L., Hammaker L. E., Marver H. S. Soluble -aminolevulinic acid synthetase of rat liver. II. Studies related to the mechanism of enzyme action and hemin inhibition. J Biol Chem. 1972 Jul 10;247(13):4132–4137. [PubMed] [Google Scholar]
- Scholnick P., Marver H. S., Schmid R. Erythropoietic protoporphyria: evidence for multiple sites of excess protoporphyrin formation. J Clin Invest. 1971 Jan;50(1):203–207. doi: 10.1172/JCI106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Johnson J. A., Stephenson B. D., Anderson A. S., Edmondson P. R., Fusaro R. M. Erythropoietic defects in protoporphyria: a study of factors involved in labelling of porphyrins and bile pigments from ALA- 3 H and glycine- 14 C. J Lab Clin Med. 1971 Sep;78(3):411–434. [PubMed] [Google Scholar]
- Shanley B. C., Zail S. S., Joubert S. M. Effect of ethanol on liver delta-aminolaevulinate synthetase in rats. Lancet. 1968 Jan 13;1(7533):70–71. doi: 10.1016/s0140-6736(68)90070-6. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Granick S. Heme control on the synthesis of delta-aminolevulinic acid synthetase in cultured chick embryo liver cells. Ann N Y Acad Sci. 1975 Apr 15;244:509–520. doi: 10.1111/j.1749-6632.1975.tb41551.x. [DOI] [PubMed] [Google Scholar]
- Sinclair P., Gibbs A. H., Sinclair J. F., de Matteis F. Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem J. 1979 Mar 15;178(3):529–538. doi: 10.1042/bj1780529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. A., Curl F. D., Valsamis M., Tschudy D. P. Abnormal iron and water metabolism in acute intermittent porphyria with new morphologic findings. Am J Med. 1972 Dec;53(6):784–789. doi: 10.1016/0002-9343(72)90197-0. [DOI] [PubMed] [Google Scholar]
- Stein J. A., Tschudy D. P. Acute intermittent porphyria. A clinical and biochemical study of 46 patients. Medicine (Baltimore) 1970 Jan;49(1):1–16. [PubMed] [Google Scholar]
- Stein J. A., Tschudy D. P., Corcoran P. L., Collins A. Delta-aminolevulinic acid synthetase. 3. Synergistic effect of chelated iron on induction. J Biol Chem. 1970 May 10;245(9):2213–2218. [PubMed] [Google Scholar]
- Stein J., Berk P., Tschudy D. A model for calculating enzyme synthetic rates during induction: application to the synergistic effect of ferric citrate on the induction of hepatic delta-aminolevulinic acid synthetase. Life Sci. 1969 Oct 15;8(20):1023–1031. doi: 10.1016/0024-3205(69)90453-6. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudilovsky O., Pestana A., Hinderaker P. H., Pitot H. C. Cyclic adenosine 3',5'-monophosphate during glucose repression in the rat liver. Science. 1971 Oct 8;174(4005):142–144. doi: 10.1126/science.174.4005.142. [DOI] [PubMed] [Google Scholar]
- TSCHUDY D. P., WELLAND F. H., COLLINS A., HUNTER G., Jr THE EFFECT OF CARBOHYDRATE FEEDING ON THE INDUCTION OF DELTA-AMINOLEVULINIC ACID SYNTHETASE. Metabolism. 1964 May;13:396–406. doi: 10.1016/0026-0495(64)90113-1. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Wagner G., Sedman R., Piper W. Effects of metals on heme biosynthesis and metabolism. Fed Proc. 1978 Jan;37(1):35–39. [PubMed] [Google Scholar]
- Tschudy D. P., Bonkowsky H. L. A steady state model of sequential irreversible enzyme reactions. Mol Cell Biochem. 1973 Nov 15;2(1):55–62. doi: 10.1007/BF01738678. [DOI] [PubMed] [Google Scholar]
- WELLAND F. H., HELLMAN E. S., GADDIS E. M., COLLINS G., HUNTER G. W., Jr, TSCHUDY D. P. FACTORS AFFECTING THE EXCRETION OF PORPHYRIN PRECURSORS BY PATIENTS WITH ACUTE INTERMITTENT PORPHYRIA. I. THE EFFECT OF DIET. Metabolism. 1964 Mar;13:232–250. doi: 10.1016/0026-0495(64)90103-9. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Dhar G. J., Bossenmaier I., Cardinal R., Petryka Z. J. Effect of hematin in acute porphyric relapse. Ann Intern Med. 1973 Jul;79(1):80–83. doi: 10.7326/0003-4819-79-1-80. [DOI] [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]
- Whiting M. J., Granick S. Delta-Aminolevulinic acid synthase from chick embryo liver mitochondria. I. Purification and some properties. J Biol Chem. 1976 Mar 10;251(5):1340–1346. [PubMed] [Google Scholar]
- Whiting M. J. Synthesis of delta-aminolaevulinate synthase by isolated liver polyribosomes. Biochem J. 1976 Aug 15;158(2):391–400. doi: 10.1042/bj1580391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Carver G. T. Action of cobalt chloride on the biosynthesis, degradation, and utilization of heme in fetal rat liver. Drug Metab Dispos. 1977 Sep-Oct;5(5):487–492. [PubMed] [Google Scholar]
- Zähringer J., Konijn A. M., Baliga B. S., Munro H. N. Mechanism of iron induction of ferritin synthesis. Biochem Biophys Res Commun. 1975 Jul 22;65(2):583–590. doi: 10.1016/s0006-291x(75)80186-0. [DOI] [PubMed] [Google Scholar]


