Abstract
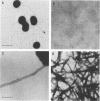
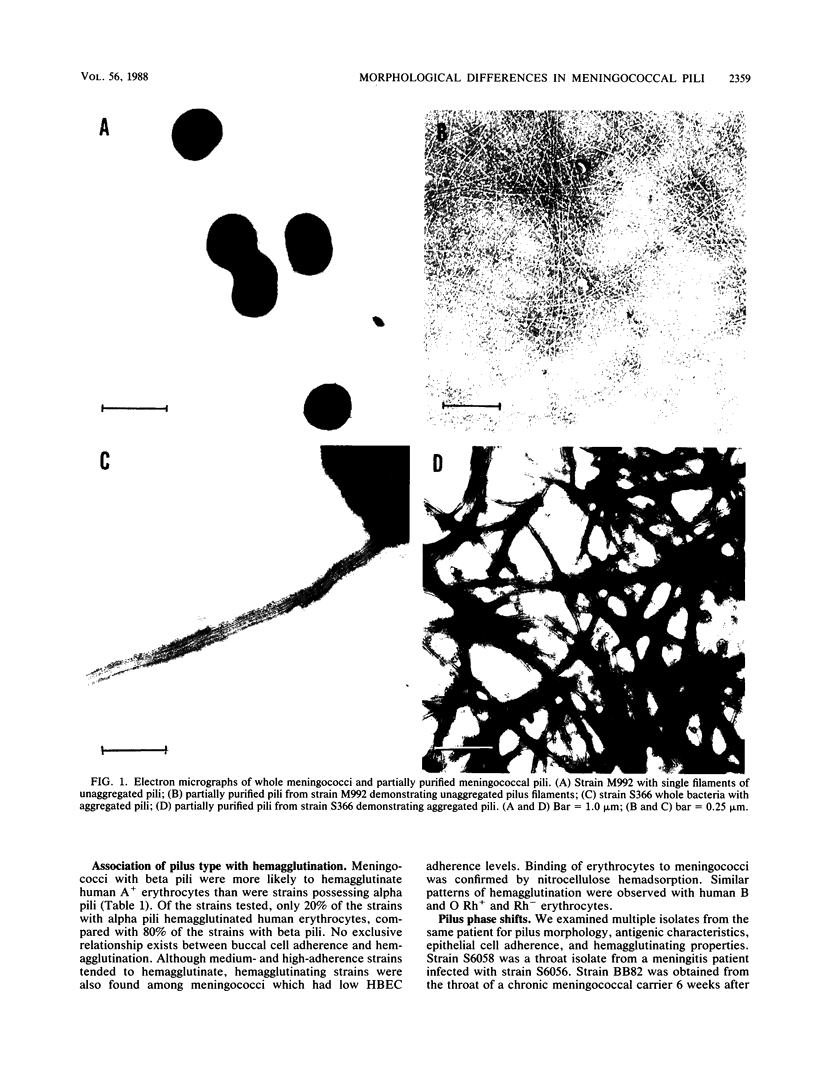
Disease and carrier isolates of Neisseria meningitidis were examined for their ability to adhere to human buccal epithelial cells and human cell lines and to hemagglutinate human erythrocytes, properties thought to be associated with the presence of pili. Seventy percent (7 of 10) of carrier isolates were found to be highly adherent to human buccal epithelial cells and to agglutinate human A, B, O, Rh-, and Rh+ erythrocytes. In contrast, 60% of the disease isolates adhered poorly to human buccal epithelial cells and 80% failed to agglutinate human erythrocytes. No adherence of either disease or carrier isolates was observed when several human cell lines were tested. When the meningococcal strains were examined by electron microscopy, 7 of 10 disease isolates were found to possess large bundles of aggregated pili (alpha-type pili), while 7 of 10 carrier isolates were found to have numerous unaggregated pili (beta-type pili). A monoclonal antibody against meningococcal pili and one against gonococcal pili reacted with 6 of 10 piliated carrier isolates and 4 of 10 piliated disease isolates. These results suggest that meningococci, like gonococci, possess different types of pili which differ in morphological, antigenic, and binding properties. In addition, antigenic and morphological differences between pili from carrier and disease isolates were observed as well as differences in adherence and hemagglutinating properties.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Ultrastructure of pili and annular structures on the cell wall surface of Neisseria meningitidis. Infect Immun. 1974 Oct;10(4):872–876. doi: 10.1128/iai.10.4.872-876.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Simonsen T., Spanne O., Lund V., Bjorvatn B. Isolates of Neisseria meningitidis from different sites in the same patient: phenotypic and genomic studies, with special reference to adherence, piliation, and DNA restriction endonuclease pattern. J Infect Dis. 1984 Sep;150(3):389–396. doi: 10.1093/infdis/150.3.389. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morita I., Masujima T., Yoshida H., Imai H. Enrichment and high-performance liquid chromatography analysis of tryptophan metabolites in plasma. Anal Biochem. 1981 Nov 15;118(1):142–146. doi: 10.1016/0003-2697(81)90170-6. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunogenicity of meningococcal antigens as detected in patient sera. Infect Immun. 1983 Apr;40(1):398–406. doi: 10.1128/iai.40.1.398-406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C., Dainelli B., Ricchetti M. Piliated Bacteroides fragilis strains adhere to epithelial cells and are more sensitive to phagocytosis by human neutrophils than nonpiliated strains. Infect Immun. 1984 Jan;43(1):189–194. doi: 10.1128/iai.43.1.189-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E. Hemagglutination by Neisseria meningitidis. Can J Microbiol. 1981 Jun;27(6):586–593. doi: 10.1139/m81-089. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Rothbard J., Schoolnik G. K. Pili of Neisseria meningitidis. Analysis of structure and investigation of structural and antigenic relationships to gonococcal pili. J Exp Med. 1985 Jun 1;161(6):1539–1553. doi: 10.1084/jem.161.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Barrera O. Gonococcal pilus subunit size heterogeneity correlates with transitions in colony piliation phenotype, not with changes in colony opacity. J Exp Med. 1983 Nov 1;158(5):1459–1472. doi: 10.1084/jem.158.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J Exp Med. 1972 Nov 1;136(5):1258–1271. doi: 10.1084/jem.136.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Wray G. P., Hitchcock P. J. Arrangement of pili in colonies of Neisseria gonorrhoeae. J Bacteriol. 1984 Jul;159(1):312–320. doi: 10.1128/jb.159.1.312-320.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Gillespie R. M., Bhatti A. R., White L. A. Differences in the adhesive properties of Neisseria meningitidis for human buccal epithelial cells and erythrocytes. Infect Immun. 1983 Jul;41(1):106–113. doi: 10.1128/iai.41.1.106-113.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]