Abstract
LKB1 plays the role of tumor suppressor, opposite to Akt, by negatively regulating mTOR through the activation of AMPK and TSC signaling. We have discovered a novel, potentially oncogenic role for LKB1, as a supporter of Akt-mediated phosphorylation of pro-apoptotic proteins. We found that Akt activation led to increased phosphorylation of FoxO3a at threonine 32 (Thr32) in LKB1 wild-type cells, but not in LKB1-null cells. Depletion of LKB1 in the cells with wild-type LKB1 resulted in attenuation of that phosphorylation of FoxO3a by activated Akt, while the restoration of LKB1 function in LKB1-null cells re-established Akt-mediated FoxO3a phosphorylation. Upon expanding our analysis to other Akt targets, using isogenic LKB1 knockdown cell line pairs and a phospho-specific antibody microarray, we observed that there was a requirement for LKB1 in the phosphorylation of other Akt down-stream targets, including Ask1 (Ser83), Bad (Ser136), FoxO1 (Ser319), FoxO4 (Ser197) and GSK3β (Ser9). Because the phosphorylation of these sites by Akt suppresses apoptosis, the requirement of LKB1 suggests that LKB1 may have an anti-apoptotic role in tumor cells with constitutively active Akt. Indeed, we found the suppression of LKB1 expression led to apoptosis in three cell lines in which Akt is constitutively active, but not in two cell lines without Akt activation. This observation may explain the lack of LKB1 somatic mutations in brain, breast and colon cancers, where Akt is frequently activated due to mutations in PI3K, PTEN or Akt itself.
Keywords: LKB1, PI3K/Akt signaling, tumor suppressor, apoptosis
Introduction
LKB1 is a serine/threonine kinase gene located on chromosome 19p13.3 (1). Inherited mutations in LKB1 give rise to Peutz-Jeghers syndrome, a disorder characterized by benign hamartomas of the GI tract and a predisposition to certain cancers (2). In addition, somatic mutation analyses indicate that bi-allelic inactivation of LKB1 is present in approximately 30% of non-small cell lung cancer (NSCLC) primary tumors and cell lines (3–6). Recent progress on the function of LKB1 places this protein at the apex of a novel signaling pathway that ultimately serves to inhibit the activity of mammalian target of rapamycin (mTOR) kinase, a key mediator of phosphatidylinositol-3 kinase (PI3K)/Akt driven survival signals (7). LKB1 is linked to mTOR regulation through the sequential activation of AMP-activated protein kinase (AMPK) and the tumor suppressor TSC2, a GTPase-activating protein that negatively regulates mTOR through the small G-protein Rheb (8). Together LKB1, AMPK and TSC2 constitute a cell stress pathway that counteracts PI3K/Akt signaling, thus suppressing mTOR-related translation under energy stress.
Though extensive genetic and functional evidence suggest that LKB1 is a tumor suppressor (2), the biological properties associated with LKB1 deficiency are complex. Some studies in model organisms suggest that inactivation of LKB1 may not always be compatible the activation of certain oncogenic signaling. For example, in mouse embryonic fibroblast (MEF) cells, a LKB1 deficiency leads to resistance to Ha-Ras transformation via a p19arf/p53-independent growth inhibitory pathway, suggesting that the early loss of LKB1 function may render cells resistant to subsequent oncogene-induced transformation (9). In Xenopus and mouse MEF cells, LKB1 depletion leads to decreases in GSK3β phosphorylation at serine 9 and subsequent down-regulation of WNT signaling (10). This data is of particular interest because the Ser9 of GSK3β is a target of aberrantly activated Akt in many human cancers, which suggests that LKB1 may be required for the phosphorylation of Akt down-stream targets. This would constitute a previously unknown pro-oncogenic role for LKB1. In this study, we directly tested the role of LKB1 in Akt signaling. Our results showed that the presence of LKB1 is required for Akt-mediated phosphorylation of the pro-apoptotic proteins.
Materials and Methods
Materials
2-deoxyglucose (2-DG) was purchased from Sigma (cat#D-8375). Mouse monoclonal antibody against LKB1 was purchased from Abcam (cat#ab15095-100). Antibodies against AMPK, phospho-AMPKα (Thr172) Akt, phospho-Akt (p-Akt, Ser473), phospho-Bad (p-Bad, Ser136), phospho-GSK3β (p-GSK3β, Ser9), p21, p27, cyclin D1 and caspase-3 were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Antibodies against FoxO3a (cat#07-702) and phospho-FoxO3a (Thr32, cat#07-695) were purchased from Upstate (Lake Placid, NY, USA). Rabbit polyclonal antibody against Bim-1 was purchased from Affinity BioReagents (Golden, CO, USA). Rabbit polyclonal anti-actin antibody was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The NSCLC cell lines H1792, A549, H23, H157, H460, H1299, H1650, H1703, HCC827, H520 and colorectal cancer cell line HCT116 were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and were propagated according to the conditions recommended by ATCC.
Immunoblot analysis
The procedures for the preparation of whole cell protein lysates and for immunoblot were as described previously (5). Whole cell protein lysates (20 µg/lane) were processed for immunoblot analysis using antibodies against specified proteins. The same blots were used in probing for phospho-specific antibodies and antibodies against total protein. Actin served as a loading control.
Selection of cells Transient siRNA Treatment
LKB1 siRNA duplexes were purchased from Dharmacon (Lafeyette, CO, USA). To control for any non-specific off-target effects of the siRNA transfection, the company’s Lamin A/C siControl was also employed. The LKB1 siRNA sequence was 5'-GGACUGACGUGUAGAACAATT-3'. Gene silencing was achieved by transfecting cells with siRNAs delivered by oligofectamine reagent (Invitrogen), according to the manufacturer's recommendation. Briefly, cells were grown to ~60–70% confluence. Oligofectamine reagent was incubated with Opti-MEM1 reduced serum medium for 10 minutes and then a mixture of siRNA was added. After incubating 15 minutes at room temperature, the siRNA mixture was diluted with medium and added to each well of cells. We used 200 pmol of siRNA per 2 ml of medium. To improve gene silencing, we transfected the same cells 48 hours after the first transfection. Twenty-four hours after their second transfection, cells were washed, then resuspended in new culture media in the presence or absence of 2-DG for a given period of time. Total cell lysates were used in the immunoblot blot analysis described above.
Adenovirus infection
NSCLC cell lines were infected with adenovirus as described previously (5). Briefly, AdEasy-GFP-LKB1 plasmid was first transfected into 293 cells for the generation of Adenovirus containing GFP-LKB1. Adeno-GFP virus was a gift from Dr. Lily Yang (Emory University). H157 was infected with either GFP-LKB1 or GFP-only adenovirus at 20 MOI for 24 hours. The infection rate of the cells was approximately 90% as determined by GFP expression.
LKB1 stable knock-down using Lentiviral shRNA
Five pre-made lentiviral LKB1 shRNA constructs and a negative control construct that was created in the same vector system (pLKO1) were purchased from OpenBiosystems (Cat# RHS3979 and RHS4078). Lentiviral helper plasmids (pCMV-dR8.2 dvpr and pCMV-VSV-G) were obtained from addgene (cat#8455 and #8454). Transient lentivirus stocks were prepared following the manufacturer’s protocol. Briefly, 1.5×106 293T cells were plated in 10 cm dishes. Cells were co-transfected with shRNA constructs (3 µg) together with 3 µg pCMV-dR8.2 dvpr and 0.3 µg pCMV-VSV-G helper constructs. Two days later, viral stocks were harvested from the culture media, which was filtered to remove non-adherent 293T cells. To select for the NSCLC cells that were stably expressing shRNA constructs, cells were plated at subconfluent densities and infected with a cocktail of 1 mL virus-containing media, 3 mL regular media, and 8 µg /mL polybrene. Selection with 0.5–2µg /mL puromycin was started 48 hours after lentivirus infection. After several weeks of selection (two weeks for H1299 and H1703 or four for H1650, HCC827 and H520), monolayers of stably-infected pooled clones were harvested for use and cryopreserved.
Cell proliferation assay
LKB1 shRNA knockdown stable cells (H1650/LKB1shRNA, H1299/ LKB1shRNA, H1703/ LKB1shRNA) and their corresponding controls (H1650/PLKO.1, H1299/PLKO.1, H1703/PLKO.1) were seeded in 96-well cell culture plates at a density of 2,000 cells per well. The attached cells were fixed in situ with addition of cold 10% trichloroacetic acid (TCA) solution at day 1, 2, 3, 4, 5 and 6. Cell number (absorbance) was then estimated by the sulforhodamine B (SRB) assay, as previously described (11). Fold values for each cell proliferation were calculated by comparison with the first day’s cell absorbance.
Colony formation assay
H1650 or H1299 cells were plated, at a density of 2×105 cells per well, in 6-well plates overnight. The following day, cells were transfected in triplicate with lipofectamine 2000 using either LKB1shRNA or the negative control pLKO.1 plasmid. For H1299 cells, 2 µg of plasmid and 6 µl of lipofectamine were used for each well. Cells were selected with 2 µg/ml of puromycin 72 hrs after transfection for 2 weeks. For H1650 cells, cells were transfected with 0.5 µg of plasmid and 1.5 µl of lipofectamine. Cells were selected with 0.5 µg/ml of puromycin 72 hrs after transfection for 4 weeks. Medium was changed every 4 days. Finally, the cells were fixed with 10% trichloroacetic acid (TCA) solution and stained with 0.5% crystal violet. Colony numbers were assessed visually and colonies containing more than 50 normal appearing cells were counted. Statistical differences in colony numbers between LKB1shRNA or pLKO.1 plasmid transfected cells were calculated using the two-sided student’s t test.
Phospho-specific Protein Microarray analysis
Phospho-specific Protein Microarray was obtained from Full Moon Biosystems, Inc. Protein microarray analysis was carried out using the protocol provided. Briefly, 100 µg of cell lysate in 50 µl of reaction mixture was labeled with 1.43 µl of Biotin in 10 µg/µl DMF (N,N-Dimethyformamide). The resulting Biotin-labeled proteins were diluted 1:20 in Coupling Solution before applying to the array for conjugation. To prepare the Antibody Microarray, it was first blocked with blocking solution for 30 minutes at room temperature, rinsed with Milli-Q grade water for 3 minutes, then dried with compressed nitrogen; finally, the array was incubated with the Biotin-labeled cell lysates at 4°C overnight. After the array slide was washed three times with 60 ml of 1× Wash Solution for 10 minutes each, the conjugated labeled protein was detected using Cy3-streptavidin.
Cell Cycle analyses
2×105 of H1650/PLKO.1 or H1650/ LKB1shRNA cells were seeded in 6-well cell culture plates. Only the live cells were harvested 4 days after cell seeding. Cells were washed with PBS and fixed in 70% ethanol overnight. Cells were then washed twice with PBS and stained using a PI/RNase staining kit (BD Pharmingen, San Jose, CA, USA) for 30 minutes at room temperature in the dark. Cell cycle analysis was carried out using a FACScan (Becton Dickinson, San Jose, CA) and FlowJo software version 7.2. A total of 10,000 cells were collected for each sample for analysis.
Apoptosis analyses
Apoptosis was measured using the Annexin V-PE Apoptosis Detection kit (BD Pharmingen, San Jose, CA) followed by flow cytometry. 2×105 of H1650/PLKO.1, H1650/ LKB1shRNA cells were seeded in 6-well cell culture plates. Both floating and attached cells were collected 4 days after cell seeding, washed twice with cold PBS and suspended in 1× binding buffer. A 100µl aliquot of the cell suspension (representing 5 × 105 cells) was transferred to a culture tube, to which 5µl of Annexin V-PE and 5µl of 7-AAD were added, and the mix incubated for 15 minutes at room temperature in the dark. Apoptosis analysis was carried out using a FACScan (Becton Dickinson, San Jose, CA) and FlowJo software version 7.2. A total of 10,000 cells were collected for each sample for analysis.
Results
Phosphorylation of FoxO3a at threonine 32 by Akt requires LKB1
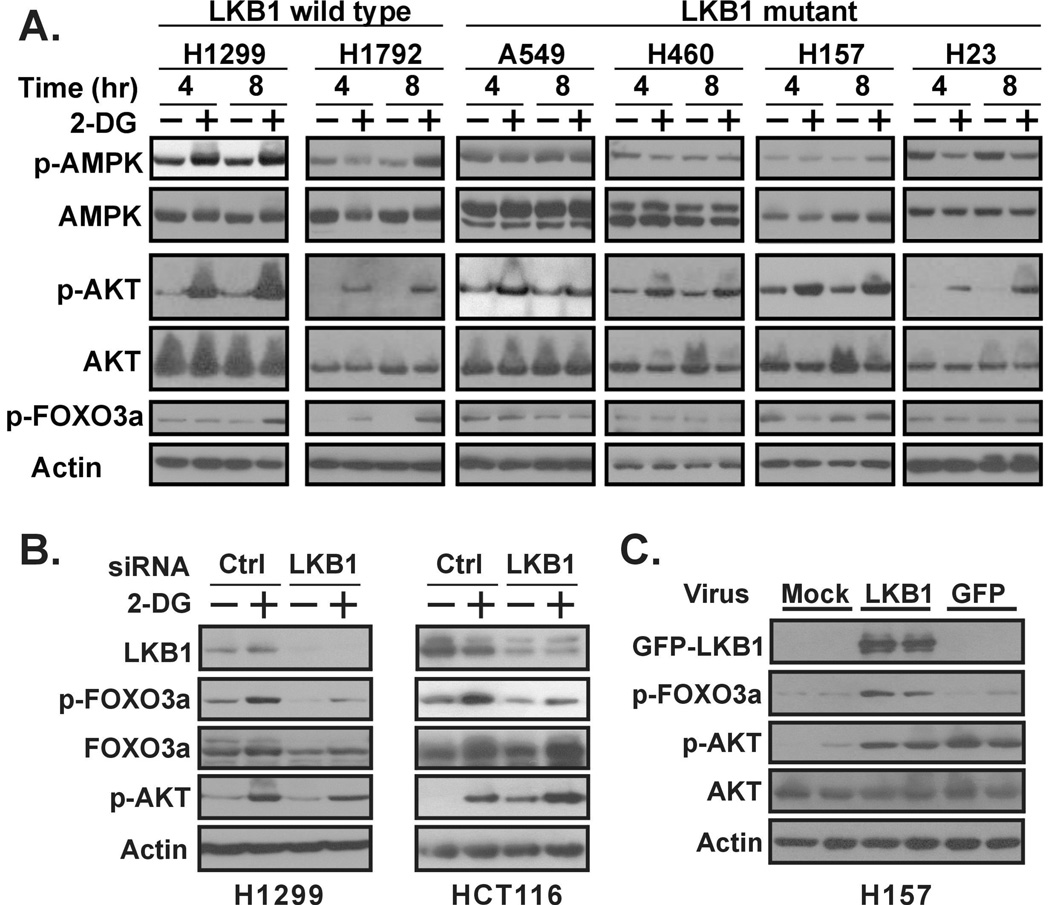
When 2-deoxyglucose (2-DG) inhibits glycolysis, it eventually leads to a decrease of intracellular ATP and an increase of intracellular AMP. In the presence of excess AMP, AMPK will undergo a conformational change upon binding AMP, which allows LKB1 to access and phosphorylate AMPK’s threonine residue 172 (Thr172), the activation site of AMPK kinase activity (12, 13). Hence, the functional status of LKB1 in NSCLC cells could be monitored by examining the phosphorylation status of AMPK Thr172 after 2-DG treatment (Figure 1A). As expected, 2-DG treatment augmented the phosphorylation of AMPK Thr172 only in LKB1 wild-type cells (H1299 and H1792), but not in LKB1-null cells (A549, H460, H157 and H23).
Figure 1. Phosphorylation of Foxo3a at Thr32 requires LKB1.
(A) An increase of FOXO3a, but not Akt phosphorylation by 2-DG treatment, is associated with the LKB1 gene status in human NSCLC cells. The indicated cells lines were treated with 25 mM 2-DG for the given times, then whole cell protein lysates were prepared. The indicated proteins were detected by immunoblot analysis. AMPK Thr172 phosphorylation status was used to determine the functional status of LKB1. (B) Inactivation of LKB1 by siRNA. H1299 and HCT116 cells were transfected with control or LKB1 siRNA. Transfected cells were treated with 2-DG (25 mM) for 8 hrs, and then whole cell protein lysates were prepared and run by immunoblot analysis. (C) Activation of LKB1 function in LKB1-null H157 cells. H157 cells were infected with Adeno-GFP-LKB1 or Adeno-GFP virus for 24 hrs, after which whole cell protein lysates were prepared. The indicated proteins were detected by immunoblot blot analysis.
We recently discovered that 2-DG also activates Akt function via a mechanism that is PI3 kinase-dependent, but LKB1-independent (14). A hyperphosphorylation of Akt can be detected as early as 15 minutes after 2-DG treatment. In fact, robust increases in Akt phosphorylation were observed in most NSCLC cell lines at 4 and 8 hrs after 2-DG treatment [Figure 1A and ref (14) ]. The pro-apoptotic transcription factor FoxO3a is a down-stream target of Akt kinase activity (15). Active FoxO3a promotes apoptosis through the transcription of Bim-1, a pro-apoptotic gene. Akt can suppress FoxO3a function via direct phosphorylation of three target sites on the molecule, including the Thr32 of FoxO3a, which has a consensus Akt target sequence (RPRSCpT) (16, 17). Consistent with this, 2-DG treatment led to a subsequent increase in FoxO3a phosphorylation in the LKB1 wild-type H1299 and H1792 cells. While a mild increase in FoxO3a phosphorylation at Thr32 was detected 4 hrs after 2-DG treatment, significant increases were not observed until 8 hrs post 2-DG treatment [Figure 1A and ref (14) ]. Interestingly, Akt-mediated phosphorylation of FoxO3a (Thr32) was not detected in LKB1 mutants (A549, H460, H23 and H157). These data suggest that the induction of Thr32 phosphorylation of FoxO3a by Akt requires a functional LKB1.
To directly address the role of LKB1 in Akt-mediated phosphorylation of Foxo3a, we determined whether the transient depletion of LKB1 in LKB1-wild-type H1299 or HCT116 cells would attenuate FoxO3a phosphorylation in response to Akt activation. We used an LKB1-siRNA that was previously designed to transiently suppress the expression of LKB1 (14). Treatment with this LKB1 siRNA resulted in an 80–90% reduction in LKB1 protein, while as expected, no reduction of LKB1 was detected with control siRNA (Figure 1B, comparing lanes 3, 4 with lanes 1, 2 for H1299 and lanes 7, 8 with lanes 5, 6 for HCT116). The transient depletion of LKB1 did not alter the 2-DG induced phosphorylation of Akt, consistent with our previous observation that 2-DG induced Akt activation does not require LKB1 (14). The levels of 2-DG induced FoxO3a phosphorylation 8 hrs after the addition of 25 mM 2-DG; however, were significantly reduced in both H1299 and HCT116 cells when LKB1 expression was down-regulated. These data demonstrated that LKB1 is required for Thr32 phosphorylation of FoxO3a by Akt.
We also determined whether the restoration of LKB1 function in LKB1-null cells could re-establish Akt phosphorylation of the Thr32 of FoxO3a. We chose an adenovirus-based system to express wild-type GFP-LKB1 in LKB1-null H157 cells. Previously, we showed that this construct is capable of restoring the phosphorylation of AMPK (Thr172) under energy stress condition (5). In our LKB1-restoration experiment, the GFP-LKB1 protein was detected only when our H157 cells were infected with adeno-LKB1 virus, but not with a control adeno-GFP virus (Figure 1C). It is known that adenovirus infection process activates Akt (18); therefore, we found that the total Akt protein levels remained the same and that infection with both the adeno-LKB1 and adeno-GFP viruses resulted in significant elevation of Akt phosphorylation at Ser473 (Figure 1C, lanes 3–6). In contrast, significant elevation of FoxO3a phosphorylation at Thr32 was only observed in those cells infected with adeno-LKB1 virus (Figure 1C, lanes 3–4), but not adeno-GFP virus (Figure 1C, lanes 5–6). Our data indicated that whereas adenoviral infection does induce Akt activation, the subsequent induction of FoxO3a phosphorylation at Thr32 required the presence of LKB1.
Depletion of LKB1 protein in H1650 cells leads to decreased cell proliferation
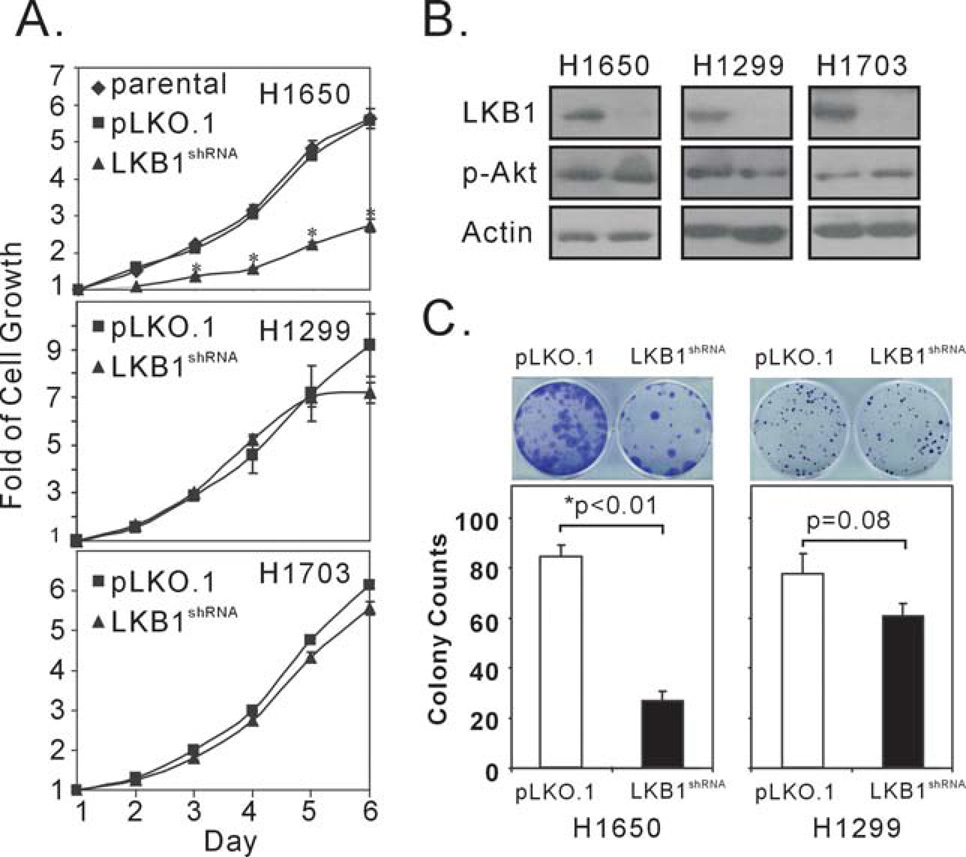
Considering our findings that LKB1 affected Akt-mediated phosphorylation of Thr32 of FoxO3a, we sought to determine the consequences of LKB1 knock-down in the context of Akt activation. H1650 has a constitutively high level of Akt phosphorylation due to the mutational activation of its EGFR, and its Akt phosphorylation can not be further induced by 2-DG treatment (14). In contrast, 2-DG induces significant elevation of Akt phosphorylation in H1299 and H1703 cells, suggesting that these two cell lines do not have aberrantly activated Akt (Figure 1A and data not shown). All three cell lines have wild-type LKB1, and we used LKB1shRNA lentivirus and puromycin to select for stable pools of cells with LKB1 depletion. The selection of stable H1650/LKB1shRNA cells took over a month, and we observed that the H1650/ LKB1shRNA cells grew slowly and experienced extensive cell death. By comparison, puromycin selection of stably infected pools of H1650/pLKO.1, H1299/LKB1shRNA,H1299/pLKO.1, H1703/LKB1shRNA, or H1703/pLKO.1 cells was completed within two weeks. When stable cell pools were analyzed for LKB1 protein expression by immunoblot, we found that LKB1 protein expression was significantly suppressed in all cell lines selected with LKB1shRNA lentivirus, but not with the control pLKO.1 virus (Figure 2B). An SRB assay was used to evaluate the impact of LKB1 on the rates of cell proliferation in all LKB1 knock-down cells (Figure 2A): While LKB1 knock-down had no effect on H1299 or H1703 cell proliferation, the depletion of LKB1 in H1650 cells significantly impeded cell growth (Figure 2A). In addition, we carried out colony formation assays in H1650 and H1299 cells transfected with either the LKB1shRNA plasmid or a control pLKO.1 plasmid. Transfection with either plasmid in H1299 cells did not result in significant differences in colony numbers (Figure 2C). In contrast, transfection with LKB1shRNA plasmid in H1650 cells significantly inhibited colony forming ability, relative to the transfection with the pLKO.1 plasmid. Because H1650 cells are sensitive to lipofectamine 2000 transfection, we also verify our findings using LKB1shRNA or pLKO.1 lentivirus in colony formation assays. Similarly, infection of H1650 cells with LKB1shRNA lentivirus significantly attenuated cell proliferation compared to infection with pLKO.1 lentivirus (supplemental Figure 1). Therefore, although down-regulation of LKB1 expression did not alter cell proliferation in H1299 and H1703 cells, it did result in a decrease in cell proliferation, specifically in the H1650 cells. In summary, we found that LKB1 depletion decreased cell proliferation only in the context of Akt activation.
Figure 2. Depletion of LKB1 in H1650 cells attenuates cell proliferation.
(A) LKB1 was depleted in H1650, H1299 and H1703 cells, and stable pools of LKB1-depleted cells were monitored for cell growth by SRB assay, using either parental cells or cells treated with pLKO.1 lentivirus as controls. Statistically significantly different points were calculated using a two-sided student’s t test and marked by an asterisk. (B) Immunoblot analyses of the LKB1 protein and phospho-Akt levels in H1650, H1299 and H1703 cells using actin as loading control. (C) Colony formation assay in H1650 cells and H1299 cells, using plasmid containing either LKB1shRNA or its vector control, pLKO.1. Values represent the mean +/− SD of quadruples. P value was calculated using student t-test.
LKB1 depletion decreases phosphorylation of other direct Akt target sites that are involved in apoptosis
To determine whether LKB1 is required for the phosphorylation of other Akt targets, a phospho-specific antibody microarray for the Akt signaling pathway was used to compare the phosphorylation status of both direct and indirect Akt targets: This antibody array includes 137 highly specific and well-characterized phospho-specific antibodies for proteins in the AKT pathway, each with six replicates (raw data included in supplemental Table 1). The paired antibodies for the same (but unphosphorylated) target sites are also included in the array, to allow determination of the relative level of phosphorylation. Because Akt is constitutively activated in H1650 cells, a comparison between Akt target proteins from H1650/LKB1shRNA cells and H1650/pLKO.1 cells enabled us to calculate a ratio for any phosphorylation changes that are due to LKB1 depletion. Using a cutoff ratio of 0.8, we identified 21 sites that were hypophosphorylated in H1650/ LKB1shRNA cells compared to H1650/pLKO.1 cells. Seven of the twenty-one sites contained the consensus target sequence for Akt phosphorylation and are known to be direct targets of Akt phosphorylation (Table 1). Therefore, the depletion of LKB1 in H1650 cells not only resulted in a decrease in FoxO3a Thr32 phosphorylation, but also attenuated the phosphorylation of other direct Akt targets, such as Ask1 (Ser83), Bad (Ser136), FoxO1 (Ser319), FoxO4 (Ser197), and Gsk3β(Ser9). To validate the antibody array results, we also directly tested the phosphorylation status of Bad (Ser136) and Gsk3β (Ser9) in the H1650/LKB1shRNA and H1650/pLKO.1 cells. As with the array, hypophosphorylation of Bad (Ser136) and Gsk3β(Ser9) was observed in H1650/LKB1shRNA cells (Figure 3A). Therefore, we confirmed that depletion of LKB1 results in decreases in the phosphorylation of multiple Akt targets.
Table 1.
Alteration of protein phosphorylation in AKT signaling pathway due to LKB1 depletion in H1650 cells.
| Consensus Akt phosphorylation taget site | RXRXXpS/T | ||||
|---|---|---|---|---|---|
| Foxo3a (Phospho-Thr32) | RPRSCpTWP | ||||
| Phosphorylation site | Ratio | Kinase involved | Biological Effects | References | |
| Decrease in phosphorylation due to LKB1 depletion | |||||
| AFX/FoxO4 (Phospho-Ser197) | 0.77 | RRRAApSMDSS | Akt | Cell survival/apoptosis | Nature 404, 782–787 |
| ASK1(Phospho-Ser83) | 0.71 | RGRGSpSVGGG | Akt | Cell survival/apoptosis | MCB 21, 893–901. |
| ASK1(Phospho-Ser966) | 0.76 | YLRSIpSLPVP | unknown | Cell survival/apoptosis | PNAS 96, 8511–8515 |
| BAD(Phospho-Ser112) | 0.58 | RSRHSpSYPAG | p90RSK, PKA | Cell survival/apoptosis | Mol Cell. 3(4):413–22 |
| BAD(Phospho-Ser136) | 0.67 | RGRSRpSAPPN | Akt | Cell survival/apoptosis | Cell 91, 231–241;Science 278, 687–689. |
| BAD(Phospho-Ser155) | 0.64 | ELRRMpSDEFV | PKA | Cell survival/apoptosis | Mol Cell.6(1):41–51 |
| BCL-2(Phospho-Ser70) | 0.61 | PVARTpSPLQT | IL3, JNK | Cell survival/apoptosis | JBC 276, 23681–23688. |
| BCL-2(Phospho-Thr56) | 0.66 | SQPGHpTPHPA | unknown | Cell survival/apoptosis | FASEB J. 16, 825–832. |
| BCL-XL(Phospho-Ser62) | 0.68 | WHLADpSPAVN | JNK | Cell survival/apoptosis | FEBS Letters, V538, 41–47 |
| c-Jun(Phospho-Ser63) | 0.68 | SDLLTpSPDVG | unknown | Cell survival/apoptosis | Nat. Genet. 21, 326–329 |
| FAK(Phospho-Tyr861) | 0.78 | NQHIpYQPVG | v-Src | FAK signaling | Biochem. Biophys. Res. Commun., 228:, 662–668. |
| FKHR/FoxO1(Phospho-Ser319) | 0.78 | RPRTSpSNAST | Akt | Cell survival/apoptosis | Mol. Cell 14, 416–418. |
| FKHRL1/FoxO3a(Phospho-Ser253) | 0.80 | RRRAVpSMDNS | Akt | Cell survival/apoptosis | Cell 96, 857–868 |
| GSK3b(Phospho-Ser9) | 0.77 | RPRTTpSFAES | Akt | Cell survival/apoptosis | Nature 378, 785–789. |
| IKKa(Phospho-Thr23) | 0.63 | RERLGpTGGFG | Akt | NFkB signaling | Nature 401(6748):82–5 |
| IRS-1(Phospho-Ser312) | 0.76 | SITATpSPASM | JNK | Insulin signaling | JBC 275(12), 9047–54 |
| p53(Phospho-Ser6) | 0.66 | MEEPQpSDPSV | CK1δ and CK1ε | u.k. | Oncogene 15, 1727–1736 |
| p53(Phospho-Ser9) | 0.72 | PQSDPpSVEPP | CK1δ and CK1ε | u.k. | Oncogene 15, 1727–1736 |
| p53(Phospho-Ser15) | 0.78 | VEPPLpSQETF | ATM,ATR, DNA-PK | DNA damage | Cell 91, 325–334; Genes Dev. 13, 152–157. |
| p53(Phospho-Thr18) | 0.66 | PLSQEpTFSDL | casein kinase 1 | DNA damage | Genes Dev. 12, 2831–2841 |
| Paxillin(Phospho-Tyr118) | 0.78 | EEHVpYSFPN | focal adhesion kinase | FAK signaling | JBC 270, 17437–17441. |
| Increase in phosphorylation due to LKB1 depletion | |||||
| 4E-BP1(Phospho-Thr45) | 1.3 | TLFSTpTPGGT | mTOR | mTOR related-Translation | Genes Dev. 13, 1422–1437 |
| c-Jun(Phospho-Ser73) | 2.2 | LLKLApSPELE | stress kinases | Cell survival/apoptosis | Nat. Genet. 21, 326–329 |
| c-Kit(Phospho-Tyr721) | 1.3 | STNEpYMDMK | unknown | activating PI3K | Nat. Genet. 24, 157–162. |
| elF4E(Phospho-Ser209) | 1.3 | ATKSGpSTTKN | MnK1 | mTOR related-Translation | MCB 19, 1871–1880.; EMBO J. 18, 270–279. |
| Gab1(Phospho-Tyr627) | 1.7 | KQVEpYLDLD | unknown | C-Met signaling | Mol. Endocrinol. 12, 914–923 |
| IKB-alpha(Phospho-Tyr42) | 1.5 | KDEEpYEQMV | unknown | NFkB signaling | |
| IRS-1(Phospho-Ser307) | 1.4 | RSRTEpSITAT | JNK, IKK | Insulin signaling | J. Clin. Invest. 107, 181–189; JBC. 277, 48115–48121. |
| IRS-1(Phospho-Ser639) | 7.0 | PMSPKpSVSAP | mTOR | Insulin signaling | PNAS 98, 4640–4645 |
| Met(Phospho-Tyr1349) | 1.5 | IGEHpYVHVN | unknown | C-Met signaling | J. Cell Biol. 149, 1419–1432 |
| p70S6 Kinase (Phospho-Ser424) | 1.2 | PRTPVpSPVKF | mTOR | mTOR related-Translation | FEBS Lett. 410, 78–82.; Exp. Cell Res. 253, 100–109 |
| PDK1(Phospho-Ser241) | 2.1 | ARANpSFVGT | autophosphorylation | activating PI3K | Biochem. J. 342, 287–292. |
| S6 (Phospho-Ser235) | 1.3 | RRRLpSSLRA | S6K | mTOR related-Translation | JBC 266, 22770–22775; JBC 267, 3074–3078 |
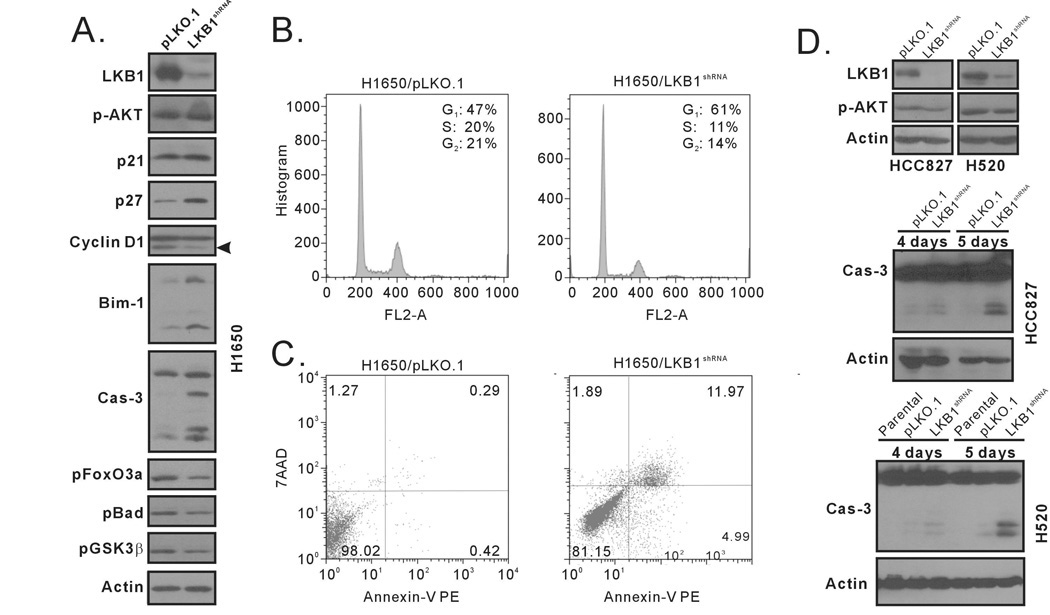
Figure 3. The depletion of LKB1 in Akt-activated cells promotes caspase-3 cleavage.
(A) Immunoblot analyses of LKB1, p-Akt, p21, p27, cyclin D1, Bim-1, caspase-3, phospho-Foxo3a (Thr32), phospho-Bad (Ser136) and phospho-GSK3β (Ser9) in H1650/LKB1shRNA or H1650/PLK.O1 cells, using actin as a loading control. (B) Cell cycle analysis of H1650/pLKO.1 and H1650/LKB1 shRNA cells. Only live cells were collected four days after cell seeding and subjected flow analysis. (C) Annexin-V and 7AAD analysis of apoptosis in H1650/LKB1-shRNA cells. Both floating and attached cells were collected four days after cell seeding and subjected to flow analysis. (D) Immunoblot analyses of caspase-3 cleavage in HCC827/LKB1shRNA and H520/ LKB1shRNA cells. Cells were seeded in 6-well plates. Both floating and attached cells were collected four or five days after cell seeding. Cell lysates were analyzed with caspase-3 antibody, with actin acting as a loading control.
Depletion of LKB1 in H1650 cells also resulted in hypophosphorylation of twelve serine/threonine sites and two tyrosine sites, all of which were not known to be direct Akt targets. Of these, Ask1 (Ser966), Bad (Ser112 and Ser155), and Bcl-2 (Ser70 and Thr56) have been shown to be involved in apoptosis. Our phospho-specific antibody microarray also evaluated eight phosphorylation sites on p53, but a change in phosphorylation was only detected from residues 6 to 18, and no alterations in phosphorylation were detected beyond Ser33. ATM can phosphorylate p53 at Ser15, and emerging evidence indicates that LKB1 does physically interact with ATM (19). Furthermore, LKB1 has been shown to directly phosphorylate p53 at Ser15 in vitro (20). Therefore, this data indicates that LKB1 may be involved in the phosphorylation of p53 at the N-terminus in vivo, as well as phosphorylation of apoptosis-related genes.
Depletion of LKB1 also resulted in hyperphosphorylation of several proteins. Using a cutoff ratio of >1.2, we identified twelve sites with increased phosphorylation. Loss of LKB1 resulted in hyperphosphorylation of several direct and indirect targets of mTOR, including 4E-BP1 (Thr45), IRS-1 (Ser639), S6 Kinase (Ser424) and S6 (Ser235). In addition, eIF4E is also involved in the mTOR-related translation process (Table 1). Because Akt and LKB1 play opposite roles in regulating mTOR kinase activity, it was expected that the depletion of LKB1 in Akt-activated H1650 cells would lead to increases in the phosphorylation of these mTOR targets. These observations of variability in phosphorylation levels induced by changes in LKB1 provided independent internal control for the validity of our antibody microarray dataset.
LKB1 depletion is associated with an increase in G1 cell cycle arrest and apoptosis in H1650 cells
Because the depletion of LKB1 in H1650 cell suppresses cell proliferation and LKB1 is required for Akt-mediated phosphorylation of apoptosis proteins and, we sought to further characterize the observed cell growth phenotype in H1650 cells. We first carried out a cell cycle analysis in H1650/ LKB1shRNA cells. We analyzed only the live cells that remained attached to tissue culture plates four days after seeding the cells. Compared to control H1650/pLKO.1 cells, the H1650/ LKB1shRNA cells displayed an increase in the percentage of cells in G1 phase (from 47% to 61%), but a decrease of those in S phase (from 20% to 11%) and G2/M phase (from 21% to 14%) (Figure 3B). The change in cell cycle profile did not appear to be related to p21, as similar levels of p21 protein were observed in both the control and LKB1 knock-down cells (Figure 3A). On the other hand, we did observe an increase in p27 and a decrease in cyclin D1 in the H1650/ LKB1shRNA cells, which suggested that the increase in G1 cells may have been related to p27-mediated cell cycle arrest.
For apoptosis assays, both the floating and attached cells were collected and analyzed four days after cell seeding. Approximately 17% of H1650/ LKB1shRNA cells underwent apoptosis. In stark contrast, only 0.7% of H1650/pLKO.1 cells were apoptotic (Figure 3C). The appearance of apoptotic cells also correlated with a significant increase in caspase 3 cleavage four days after cell seeding (Figure 3A). Consistent with LKB1 transient depletion analysis in Figure 1, the stable down-regulation of LKB1 protein expression in H1650 cells was correlated with a decrease in the phosphorylation of FoxO3a at Thr32 (Figure 3A). Unphosphorylated FoxO3a translocates to the nucleus, acting as a transcription factor to increase Bim-1 transcription (21). Indeed, we did observe an increase in total Bim-1 protein in the H1650/ LKB1shRNA cells. The activation of caspase-3 cleavage; however, was not detectable early on, only four or five days after cell seeding (data not shown).
LKB1-depletion in other NSCLC cells with aberrant Akt activation also results in increases in caspase-3 cleavage
Our data suggest that LKB1 depletion induces caspase-3 cleavage and apoptosis, but only in the context of aberrant Akt activation. To determine whether this was true in other cell lines, we also depleted LKB1 in HCC827 and H520 cells. The HCC827 NSCLC cell line harbors an exon 19 deletion (DelE746A750) of EGFR, which results in the constitutive activation of Akt (22). We generated LKB1-stable knock-down pools in this cell line. Consistent with results found for H1650 cells, we detected significant caspase-3 cleavage five days after seeding the cells (Figure 3D). Colony formation assay with LKB1shRNA plasmid in HCC827 cells also had fewer colonies than the control pLKO.1 plasmid (supplemental Figure 2). H520 cells have a somatic amplification of the PIK3CA gene (23) and we previously showed that this amplification results in elevated Akt phosphorylation (14) . LKB1-stable knock-down pools were generated for H520: LKB1 depletion also led to significant caspase-3 cleavage five days after seeding (Figure 3D). In addition, SRB analysis indicated that the depletion of LKB1 in H520 cells significantly impeded cell growth compared to either the parental cells or H520/pLKO.1 cells (supplemental Figure 3). In contrast, caspase-3 cleavage was never detected in the H1299/ LKB1shRNA and H1703/LKB1shRNA cells (data not shown). These combined data suggested that the depletion of LKB1 in NSCLC cell lines that have aberrantly activated Akt promoted apoptosis, indicating that LKB1 is required for Akt-mediated phosphorylation of pro-apoptotic proteins and that the depletion of LKB1 induces apoptosis in the context of that aberrant Akt activation.
Discussion
To date, LKB1 has been considered a tumor suppressor because the hereditary and somatic loss of function mutations of this gene are associated with an increased risk of cancer development. This idea was further supported by functional data, as it was found that the restoration of LKB1 function in LKB1-null cells leads to either apoptosis or cell cycle arrest (24, 25). Current analysis of LKB1 function has focused on its regulation of AMPK and mTOR signaling. Because LKB1/AMPK signaling inhibits mTOR, but activated Akt stimulates mTOR activity, LKB1 and Akt are thought to play opposing roles with regard to mTOR regulation (7, 8). Here, we show that LKB1 is necessary for cancer cell survival in the context of aberrant Akt activation.
The aberrant activation of Akt occurs frequently in human cancers; thus, activated Akt is thought to contribute to tumor formation partly by preventing apoptosis. Activated Akt block apoptosis through the phosphorylation and inactivation of the FoxO transcription factors, Ask1, Bad and GSK3β (15, 26). For example, FoxO3a is a transcription factor that can promote apoptosis by activating the transcription of Bim-1, a pro-apoptotic gene. Akt inhibits FoxO3a function by phosphorylating FoxO3a on Thr32, Ser253 and Ser315, which results in cytoplasmic retention of FoxO3a (27). We carried out a detailed analysis on Thr32 phosphorylation of FoxO3a and demonstrated that upregulation of FoxO3a phosphorylation at Thr32 by Akt required the function of wild-type LKB1. The necessity of LKB1 in this phosphorylation process was supported by four lines of evidence. First, we had previously shown that 2-DG can activate Akt via PI3 Kinase and that 2-DG-induced Akt activation leads to subsequent FoxO3a phosphorylation at Thr32 in LKB1 wild-type cells (14) . Concordant increases in Akt and FoxO3a phosphorylation levels; however, were not observed in four LKB1-null NSCLC cell lines (Figure 1A). In addition, we demonstrated that transient depletion of LKB1 by RNAi in LKB1 wild-type H1299 or HCT116 cells did not affect 2-DG-induced Akt phosphorylation; it led instead to a decrease in 2-DG-induced phosphorylation of FoxO3a (Figure 1B). Third, adenovirus infection, which induces Akt phosphorylation, only augmented FoxO3a phosphorylation in the LKB1-null cells that ectopically expressed LKB1 (Figure 1C). Fourth, the stable depletion of LKB1 in H1650 cells with aberrantly activated Akt also resulted in the down-regulation of FoxO3a phosphorylation (Figure 3A). Therefore, LKB1 was found to be necessary for Akt-mediated FoxO3a phosphorylation at Thr32. This is a novel finding, because it provides the first evidence that LKB1 and Akt play co-operative roles with regard to the phosphorylation of FoxO3a on Thr32.
Our antibody microarray analysis indicated that the requirement for LKB1 in the phosphorylation of Akt targets was not limited to a single Akt target. On the contrary, the depletion of LKB1 in H1650 cells resulted in decreases in the phosphorylation of Ask1 (Ser83), Bad (Ser136), FoxO1 (Ser319), FoxO4 (Ser197), and Gsk3β (Ser9). The validity of this antibody microarray analysis was supported by the upregulation of phosphorylation in the mTOR targets in the same dataset and by confirmatory immunoblot analysis of phospho-Bad and phospho-Gsk3β levels (Figure 3A). Our new data indicated that LKB1 is required for the suppression of multiple pro-apoptotic signaling molecules by an aberrantly activated Akt.
It is unknown whether LKB1 directly or indirectly participates in the phosphorylation of Akt target proteins. Direct interaction between LKB1 and Akt has not been demonstrated previously, and it is possible that LKB1 mediates this effect through its down-stream substrates. Even though LKB1/AMPK signaling is the major focus of existing literature, it is important to note that LKB1 has 11 other substrates, whose biological functions have been poorly studied to date (28). Therefore, it will be important to determine in the future whether LKB1 or its down-stream target(s) directly participate in the phosphorylation of Akt substrates.
Because Akt mediates its anti-apoptotic activity through the phosphorylation of these apoptosis-promoting molecules, the depletion of LKB1 should promote apoptosis in cancer cells with aberrantly activated Akt function. Indeed, the depletion of LKB1 enhanced caspase-3 cleavage in three NSCLC cell lines (H1650, HCC827 and H520) with activated Akt functions (Figures 3). However, LKB1 depletion did not alter the growth characteristics of two other NSCLC cell lines (H1299 and H1703) that were without Akt activation (Figure 2). Therefore, LKB1 depletion only enhanced apoptosis in those cancer cells with aberrant Akt activation. Interestingly, the depletion of LKB1 in Akt-activating cells did not result in caspase-3 cleavage immediately after cell seeding, it took several days.
If active LKB1 is required for the phosphorylation of Akt targets that are involved in apoptosis, the inactivation of LKB1 will not provide a growth advantage to cancer cells with pre-existing Akt activation. Consequently, a LKB1 mutation should not be naturally selected for in cancer cells with aberrantly active Akt function. Oncogenic activation of Akt occurs through multiple mechanisms, including mutational activation of PI3 Kinase, mutational inactivation of PTEN phosphatase or gene amplification of Akt itself (26), and is frequently observed in most solid tumor types except NSCLCs (29–33). In contrast, while somatic LKB1 mutations frequently occur in NSCLC, they are rarely observed in the other major tumor types (34–38). We suspect that the cooperation between Akt and LKB1 in the phosphorylation of these pro-apoptotic genes may be a reason that somatic LKB1 mutations are rarely observed in brain, breast and colorectal tumors: These tumors are likely to have a high frequency of PI3K activations, PTEN deletions or Akt amplifications. Because most of our studies were carried out in NSCLC cancer cell lines, our observation will require further evaluation in other cancer types.
In summary, we discovered that LKB1 is required for the phosphorylation of pro-apoptotic proteins by Akt. This is the first evidence that LKB1 plays a potentially oncogenic role in cells having activated Akt. Our data suggest that the mutational inactivation of LKB1 may not facilitate oncogenic transforming mediated by aberrant Akt activation.
Acknowledgements
We would like to thank Dr. Yaping Zong from Full moon BioSystems, Inc for his help with phospho-specific protein microarray analysis. Grant support. National Cancer Institute (P01 CA116676-030002 to W.Z., P01 CA116676-01A1 to F.K., RO1 CA 118470-01 to S-Y.S.). W.Z., F. K, and S-Y.S. are Georgia Cancer Coalition distinguished Cancer Scholars. W.Z. is an American Cancer Society Research Scholar.
References
- 1.Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 2.Yoo LI, Chung DC, Yuan J. LKB1--a master tumour suppressor of the small intestine and beyond. Nat Rev Cancer. 2002;2:529–535. doi: 10.1038/nrc843. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Cespedes M, Parrella P, Esteller M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- 4.Launonen V. Mutations in the human LKB1/STK11 gene. Hum Mutat. 2005;26:291–297. doi: 10.1002/humu.20222. [DOI] [PubMed] [Google Scholar]
- 5.Zhong D, Guo L, de Aguirre I, et al. LKB1 mutation in large cell carcinoma of the lung. Lung Cancer. 2006;53:285–294. doi: 10.1016/j.lungcan.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 7.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardeesy N, Sinha M, Hezel AF, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 10.Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5:889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Yue P, Chan CB, et al. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation. Mol Cell Biol. 2007;27:7405–7413. doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods A, Johnstone SR, Dickerson K, et al. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Zhong D, Liu X, Schafer-Hales K, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther. 2008;7:809–817. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 15.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, White LR, Clark SA, et al. Akt/protein kinase B activation by adenovirus vectors contributes to NFkappaB-dependent CXCL10 expression. J Virol. 2005;79:14507–14515. doi: 10.1128/JVI.79.23.14507-14515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes N, Sun Y, Chen S, et al. DNA damage-induced association of ATM with its target proteins requires a protein interaction domain in the N terminus of ATM. J Biol Chem. 2005;280:15158–15164. doi: 10.1074/jbc.M412065200. [DOI] [PubMed] [Google Scholar]
- 20.Zeng PY, Berger SL. LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 2006;66:10701–10708. doi: 10.1158/0008-5472.CAN-06-0999. [DOI] [PubMed] [Google Scholar]
- 21.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helfrich BA, Raben D, Varella-Garcia M, et al. Antitumor activity of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor gefitinib (ZD1839, Iressa) in non-small cell lung cancer cell lines correlates with gene copy number and EGFR mutations but not EGFR protein levels. Clin Cancer Res. 2006;12:7117–7125. doi: 10.1158/1078-0432.CCR-06-0760. [DOI] [PubMed] [Google Scholar]
- 23.Singh B, Reddy PG, Goberdhan A, et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiainen M, Ylikorkala A, Makela TP. Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc Natl Acad Sci U S A. 1999;96:9248–9251. doi: 10.1073/pnas.96.16.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karuman P, Gozani O, Odze RD, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307–1319. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 26.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 27.Arden KC. FoxO: linking new signaling pathways. Mol Cell. 2004;14:416–418. doi: 10.1016/s1097-2765(04)00213-8. [DOI] [PubMed] [Google Scholar]
- 28.Lizcano JM, Goransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forgacs E, Biesterveld EJ, Sekido Y, et al. Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene. 1998;17:1557–1565. doi: 10.1038/sj.onc.1202070. [DOI] [PubMed] [Google Scholar]
- 30.Lee JW, Soung YH, Kim SY, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–1480. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 31.Parsons R. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol. 2004;15:171–176. doi: 10.1016/j.semcdb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 33.Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene. 1998;17:475–479. doi: 10.1038/sj.onc.1201956. [DOI] [PubMed] [Google Scholar]
- 34.Sobottka SB, Haase M, Fitze G, Hahn M, Schackert HK, Schackert G. Frequent loss of heterozygosity at the 19p13.3 locus without LKB1/STK11 mutations in human carcinoma metastases to the brain. J Neurooncol. 2000;49:187–195. doi: 10.1023/a:1006442024874. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Lindblom A. Germline mutation screening of the STK11/LKB1 gene in familial breast cancer with LOH on 19p. Clin Genet. 2000;57:394–397. doi: 10.1034/j.1399-0004.2000.570511.x. [DOI] [PubMed] [Google Scholar]
- 36.Avizienyte E, Loukola A, Roth S, et al. LKB1 somatic mutations in sporadic tumors. Am J Pathol. 1999;154:677–681. doi: 10.1016/S0002-9440(10)65314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avizienyte E, Roth S, Loukola A, et al. Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res. 1998;58:2087–2090. [PubMed] [Google Scholar]
- 38.Bignell GR, Barfoot R, Seal S, Collins N, Warren W, Stratton MR. Low frequency of somatic mutations in the LKB1/Peutz-Jeghers syndrome gene in sporadic breast cancer. Cancer Res. 1998;58:1384–1386. [PubMed] [Google Scholar]