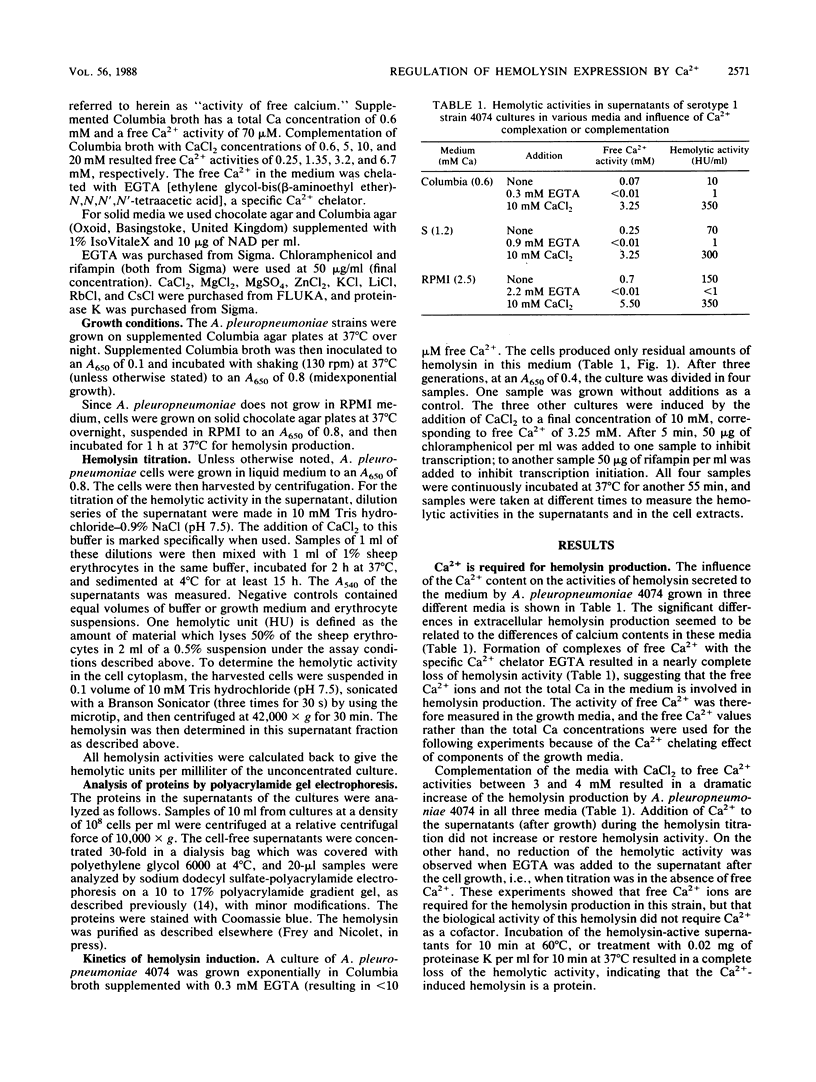
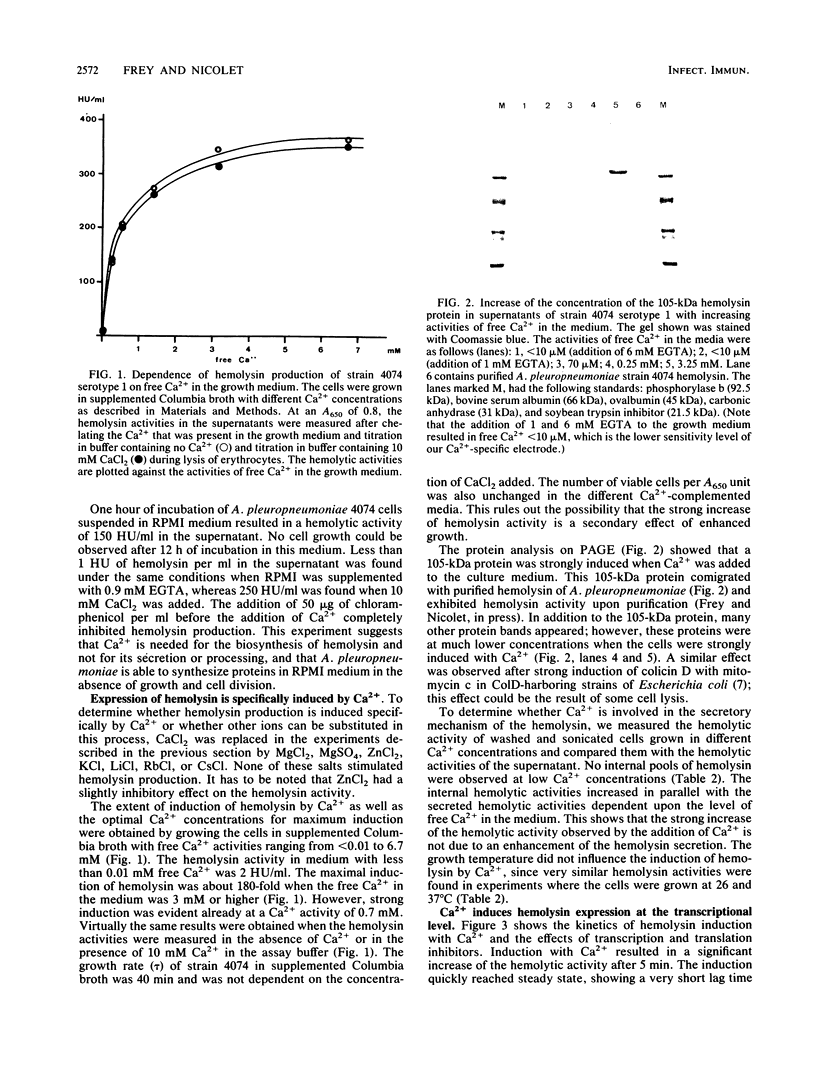
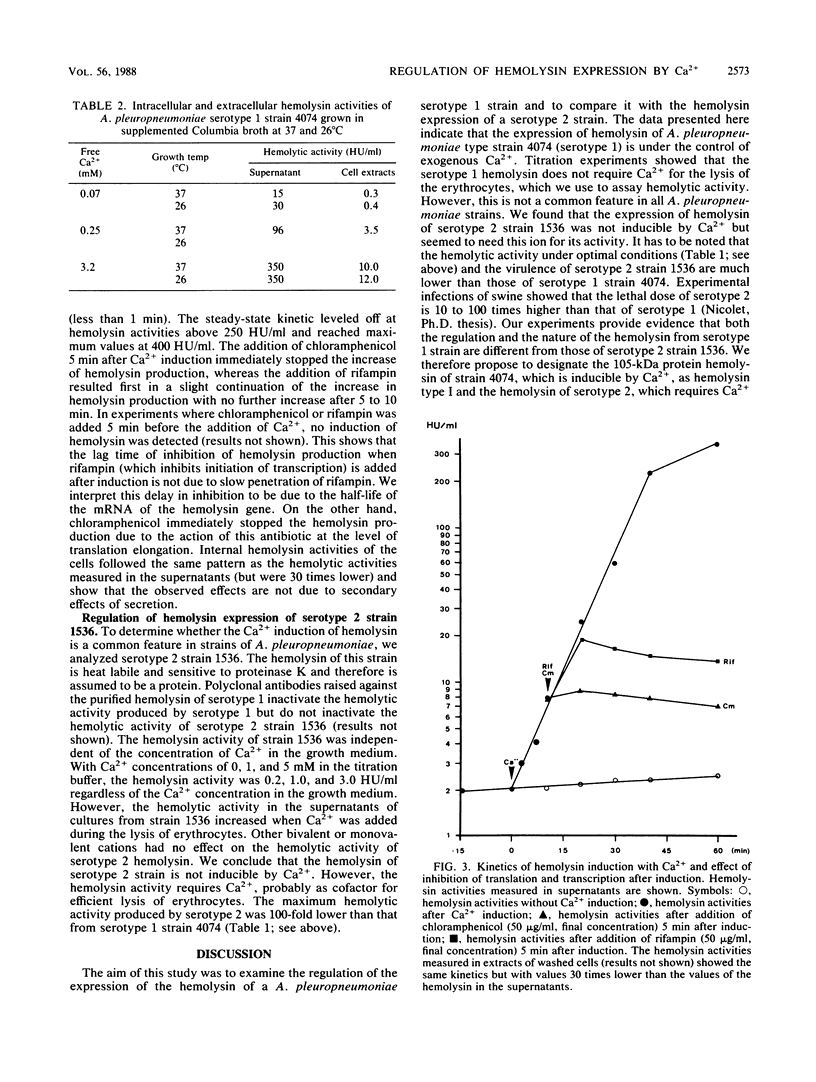
Abstract
Actinobacillus pleuropneumoniae, the causative agent of swine pleuropneumonia, secretes a hemolytic activity which is thought to be a factor involved in the pathogenesis of the disease. The biosynthesis of hemolysin by serotype 1 strain 4074 was strongly dependent on the activity of free Ca2+ in the growth medium. At activities of free Ca2+ below 50 microM, very low hemolytic activities could be detected in the growth medium and in cell extracts. Maximal hemolytic activities of up to 400 hemolytic units per ml could be measured in growth medium containing free Ca2+ activities above 3 mM. Other bivalent cations did not stimulate the production of hemolysin. Neither the growth rate nor the secretion of hemolysin was affected by increasing Ca2+ concentrations in the medium. The hemolysin of serotype 1 did not require Ca2+ as a cofactor for the lysis of erythrocytes. Ca2+ induced the expression of a 105-kilodalton protein, which was secreted. This protein comigrated with purified hemolysin on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and exhibited hemolysin activity upon purification. Inhibition experiments with rifampin suggest that the hemolysin of A. pleuropneumoniae is regulated by Ca2+ at the transcriptional level. The threshold of hemolysin induction was around 700 microM free Ca2+, a concentration which is similar to that found in blood serum. The Ca2+-inducible hemolysin represents a novel type of positively regulated bacterial gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Olander H. J. Isolation and biological characterization of two lipopolysaccharides and a capsular-enriched polysaccharide preparation from Haemophilus pleuropneumoniae. Am J Vet Res. 1986 Jul;47(7):1433–1441. [PubMed] [Google Scholar]
- Frey J., Ghersa P., Palacios P. G., Belet M. Physical and genetic analysis of the ColD plasmid. J Bacteriol. 1986 Apr;166(1):15–19. doi: 10.1128/jb.166.1.15-19.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangola P., Rosen B. P. Maintenance of intracellular calcium in Escherichia coli. J Biol Chem. 1987 Sep 15;262(26):12570–12574. [PubMed] [Google Scholar]
- Gill D. M. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982 Mar;46(1):86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. E., Bertram T. A. Morphological and biochemical comparison of virulent and avirulent isolates of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1986 Feb;51(2):419–424. doi: 10.1128/iai.51.2.419-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Nakai T., Sawata A. Interaction between heat-stable hemolytic substance from Haemophilus pleuropneumoniae and porcine pulmonary macrophages in vitro. Infect Immun. 1986 Feb;51(2):563–570. doi: 10.1128/iai.51.2.563-570.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Sawata A., Nakase Y. Haemophilus infections in chickens. I. Characterization of Haemophilus paragallinarum isolated from chickens affected with coryza. Nihon Juigaku Zasshi. 1978 Feb;40(1):65–73. doi: 10.1292/jvms1939.40.65. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin P. G., Lachance P., Niven D. F. Production of RNA-dependent haemolysin by Haemophilus pleuropneumoniae. Can J Microbiol. 1985 May;31(5):456–462. doi: 10.1139/m85-085. [DOI] [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S. Growth and hemolysin production by Haemophilus pleuropneumoniae cultivated in a chemically defined medium. Can J Microbiol. 1986 Oct;32(10):801–805. doi: 10.1139/m86-147. [DOI] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Characterization of the hemolysin produced by haemophilus pleuropneumoniae. Am J Vet Res. 1983 Feb;44(2):344–347. [PubMed] [Google Scholar]
- Pollack C., Straley S. C., Klempner M. S. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. 1986 Aug 28-Sep 3Nature. 322(6082):834–836. doi: 10.1038/322834a0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Schooley R., Dolin R., Ennis F. A., Gross P., Gwaltney J. M. Serologic responses and systemic reactions in adults after vaccination with monovalent A/USSR/77 and trivalent A/USSR/77, A/Texas/77, B/Hong Kong/72 influenza vaccines. Rev Infect Dis. 1983 Jul-Aug;5(4):748–757. doi: 10.1093/clinids/5.4.748. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Bacterial calcium transport. Biochim Biophys Acta. 1987 Apr 27;906(1):101–110. doi: 10.1016/0304-4157(87)90007-4. [DOI] [PubMed] [Google Scholar]
- SHOPE R. E. PORCINE CONTAGIOUS PLEUROPNEUMONIA. I. EXPERIMENTAL TRANSMISSION, ETIOLOGY, AND PATHOLOGY. J Exp Med. 1964 Mar 1;119:357–368. doi: 10.1084/jem.119.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]