Abstract
In animals, successful production of the visual chromophore (11-cis-retinal or derivatives thereof such as 11-cis-3-hydroxy-retinal) is essential for photoreceptor cell function and survival. These carotenoid-derived compounds must combine with a protein moiety (the opsin) to establish functional visual pigments. Evidence from cell culture systems has implicated that the retinal pigment epithelium protein of 65 kDa (RPE65) is the long-sought all-trans to 11-cis retinoid isomerase. RPE65 is structurally related to nonheme iron oxygenases that catalyze the conversion of carotenoids into retinoids. In vertebrate genomes, two carotenoid oxygenases and RPE65 are encoded, whereas in insect genomes only a single representative of this protein family, named NinaB (denoting neither inactivation nor afterpotential mutant B), is encoded. We here cloned and functionally characterized the ninaB gene from the great wax moth Galleria mellonella. We show that the recombinant purified enzyme combines isomerase and oxygenase (isomerooxygenase) activity in a single polypeptide. From kinetics and isomeric composition of cleavage products of asymmetrical carotenoid substrates, we propose a model for the spatial arrangement between substrate and enzyme. In Drosophila, we show that carotenoid-isomerooxygenase activity of NinaB is more generally found in insects, and we provide physiological evidence that carotenoids such as 11-cis-retinal can promote visual pigment biogenesis in the dark. Our study demonstrates that trans/cis isomerase activity can be intrinsic to this class of proteins and establishes these enzymes as key components for both invertebrate and vertebrate vision.
Keywords: RPE65, vision, visual chromophore
Animal visual pigments (rhodopsins) are bipartite G-protein-coupled receptors consisting of an opsin moiety and a carotenoid-derived retinylidene chromophore (1). Two fundamental issues in the pathway for visual chromophore production have resisted molecular analysis for a long time: first, the oxidative cleavage of C40 carotenoids into C20 retinoids; and second, the all-trans to 11-cis isomerization of retinoids.
The molecular basis for the oxidative cleavage was resolved by cloning and characterization of NinaB (denoting neither inactivation nor afterpotential mutant B), which converts carotenoids into retinoids in Drosophila melanogaster (2, 3). Thereafter, several related carotenoid-15,15′-oxygenases (CMO1) have been identified and characterized in vertebrates including human beings (4–7). In addition, in vertebrates a second type of carotenoid-oxygenase, carotenoid-9′,10′-monooxygenase (CMO2) has been identified that cleaves carotenoids asymmetrically (8, 9), although its physiological function is not yet fully understood.
The isomerization problem concerns the questions of how the visual chromophore is produced to establish functional visual pigments and, in vertebrates, how the all-trans visual photoproduct is isomerized back to the 11-cis chromophore, to maintain visual responsiveness. Recently, evidence in cell culture has been provided (10–12) that retinal pigment epithelium protein of 65 kDa (RPE65) catalyzes all-trans-retinyl ester conversion to 11-cis-retinol. Consistently, mutations in RPE65 cause visual chromophore deficiency in mammals (13).
Interestingly, carotenoid-oxygenases and the retinoid isomerase RPE65 belong to a family of structurally related nonheme iron oxygenases (9, 14) that was first described in plants (15). Whereas mammalian genomes encode three family members (RPE65, CMO1 and CMO2), insect genomes (such as Drosophila melanogaster, Anopheles gambiae, or Apis mellifera) encode only a single member of this class of enzyme, namely NinaB. In insects, biochemical evidence has been provided that carotenoids can promote visual chromophore production in the dark (16). However, the molecular nature of the isomerase in this pathway has remained elusive.
Here, we speculated that oxidative cleavage and isomerase activity, separate functions of vertebrate enzymes, are embedded in a single polypeptide in insects. We cloned and biochemically characterized the ninaB gene product of the great wax moth Galleria mellonella. Our biochemical analyses show that the purified recombinant enzyme catalyzes oxidative cleavage and all-trans to 11-cis isomerization of carotenoids. In Drosophila, we show that this unique enzymatic property of NinaB is more generally found in insects and that carotenoids like 11-cis-retinal can promote visual pigment biogenesis in the dark.
Results
Cloning of NinaB from Galleria mellonella.
To clone the Galleria ninaB gene, we generated oligonucleotide primers deduced from known insect ninaB cDNA sequences. By reverse transcription-polymerase chain reaction (RT-PCR), we amplified a partial cDNA from a total RNA preparation isolated from late pupal heads. With RACE-PCR, we cloned a full-length ninaB cDNA from Galleria. The gninaB gene encoded a protein of 513 aa with a MW of 57.8 kDa (supporting information (SI) Fig. S1). At the amino acid level, gNinaB shared 56% and 35% overall identity with Drosophila NinaB and human RPE65, respectively. Four His residues (His-184, His-242, His-312, His-503) as well as Asp-55 were conserved, which have been shown to be crucial for binding the cofactor ferrous iron and for enzymatic activity (12, 17).
gNinaB Catalyses Oxidative Cleavage and All-trans to 11-cis Isomerization of β,β-Carotene.
For biochemical characterization, gNinaB was expressed as a His-tagged protein in Escherichia coli. We first performed tests for enzymatic activity with crude protein extracts in the presence of 1% octyl β-D-1-thioglucopyranoside (OTG) and 16 μmol/l β,β-carotene. As shown in Fig. 1A, retinal production increased with incubation time. With short incubation times, all-trans and 11-cis-retinal existed in nearby equal molar amounts. With longer incubation, increasing amounts of 13-cis-retinal were found. This finding indicated that gNinaB catalyzed the production of 11-cis-retinal and all-trans-retinal but that the 11-cis-retinal product was thermally converted either into the all-trans or the 13-cis stereoisomer in the aqueous test solution. Therefore, we performed tests for enzymatic activity in the presence of 3% OTG to enlarge the storage capability for lipophilic products. When the micelle concentration was increased, solely all-trans- and 11-cis-retinal were present and 13-cis-retinal was no longer detectable (Fig. 1B, 1C).
Fig. 1.
Tests for enzymatic activity with gNinaB. (A) Time dependency of β,β-carotene cleavage and steroisomeric composition of the retinaldehyde products. The reaction was carried out with 6 μg gNinaB (crude protein extract), 1% OTG, and 16 μmol/l β,β-carotene at 28 °C. (B) Composition of retinal stereoisomers in tests for enzymatic activity with 6 μg gNinaB (crude protein extract) and 10 μg purified gNinaB. The reaction was carried out in the presence of 3% OTG and 20 μmol/l β,β-carotene at 28 °C for 15 minutes. The insert shows a silver stained sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) with 100 ng purified gNinaB. For values in (A) and (B), the mean ± SD of three independent experiments are given. (C) HPLC profiles at 350 nm of lipid extract from an in vitro test for enzymatic activity (boldface line) and for the authentic standards 11-cis-retinaloxim (dot-dot-dashed line) and all-trans-retinaloxim (dashed line). 1, β,β-carotene; 2, 11-cis-retinaloxim (syn); 3, all-trans-retinaloxim (syn); 4, 11-cis-retinaloxim (anti); all-trans-retinaloxim (anti). Inserts show UV/Vis spectra of peak 2, λmax 346 and peak 3, λmax 357.
To demonstrate that this carotenoid-isomerooxygenase activity is intrinsic to gNinaB, we purified the protein by affinity chromatography (Fig. 1B). Tests for enzymatic activity with the recombinant purified protein revealed that gNinaB catalyzed oxidative cleavage at the C15,C15′ double bound and an all-trans to 11-cis isomerization of one half site of β,β-carotene (Fig. 1B). Thus, both isomerase and oxygenase activities can be assigned to gNinaB.
gNinaB Converts Zeaxanthin Directly to the Visual Chromophore.
Galleria, like Drosophila, uses 11-cis-3-hydroxy-retinal as visual chromophore (18). Analysis of the carotenoid composition of pupal heads revealed that only hydroxylated carotenoids such as zeaxanthin and lutein existed in the moth (Fig. S2). This finding indicated that Galleria can directly convert hydroxylated carotenoids into the visual chromophore. Thus, we performed tests with gNinaB for zeaxanthin cleavage. As shown in Fig. S3, gNinaB catalyzed the conversion of this 3,3′-dihydroxy β,β-carotene derivative into all-trans and 11-cis-3-hydroxy-retinal.
Isomeric Composition of the Products of Symmetrical and Asymmetrical Substrates.
In addition to β,β-carotene and zeaxanthin, we determined gNinaB activity with asymmetric carotenoid substrates (lutein, α-carotene, cryptoxanthin). Lutein and α-carotene possess one β-ionone and one ε-ionone ring, whereas cryptoxanthin possesses one 3-hydroxylated and one non-hydroxylated β-ionone ring (Fig. 2). High-performance liquid chromatography (HPLC) analyses revealed that gNinaB readily converted lutein and α-carotene to the corresponding retinal derivatives (Fig. S4 and Fig. S5). As with symmetric carotenoids, 11-cis and all-trans retinoid products existed (Fig. 2). In contrast, the amount of the 11-cis-retinoid stereoisomer was unequally distributed between the two cleavage products of asymmetric carotenoids. The 11-cis configuration was exclusively present in the products with a terminal β-ionone ring (retinal and 3-hydroxy-retinal), whereas the product with the ε-ring (α-retinal, 3-hydroxy-α-retinal) exclusively existed in the all-trans configuration (Fig. 2). Analysis with cryptoxanthin, harboring two β-rings but one hydroxylated at position 3, revealed another prevalence of gNinaB (Fig. S6). With this substrate, 11-cis-3-hydroxy-retinal and 11-cis-retinal existed in a molar ratio of 8:1 (Fig. 2), indicating that gNinaB preferentially isomerized the half site of cryptoxanthin with the hydroxylated β-ionone ring.
Fig. 2.
Stereoisomeric composition of retinoid cleavage products derived from symmetric and asymmetric carotenoids. Tests for enzymatic activity were carried out with 6 μg gNinaB (crude protein extract) in the presence of 3% OTG and 20 μmol carotenoids at 28 °C for 20 min. Values given are the mean ± SD of three independent experiments.
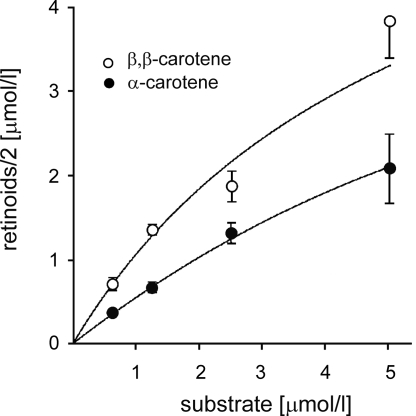
Two Binding Sites Alter the Reaction Kinetics.
Tests with asymmetric carotenoids provided evidence that gNinaB specifically interacted with one half site of carotenoid substrate. Accordingly, gNinaB should display unique kinetic properties. At low substrate concentrations, the turnover rate of a symmetric carotenoid with two β-ionone rings should significantly differ as compared with an asymmetric carotenoid with only one β-ionone ring structure. Because the amount of binding sites is doubled, k1 will be doubled while k-1 remains constant. That means the Km value should be halved compared with an asymmetric carotenoid. At saturating substrate concentrations, however, for both substrates the turnover number kcat (or Vmax) should be the same. To test this hypothesis, we compared the kinetics of β,β-carotene and α-carotene conversion by gNinaB (Fig. 3). At low substrate concentrations, the turnover of β,β-carotene was just double of that of α-carotene. GOSA fit: for β,β-carotene Km = 10.4 ± 0.63 μmol/l, turnover number kcat = 0.52 ± 0.31/min; for α-carotene Km = 22.13 ± 0.67 μmol/l, kcat = 0.49 ± 0.18/min. Thus, kinetic analysis provided additional evidence that gNinaB specifically interacted with one half site of the carotenoid substrate molecule.
Fig. 3.
Kinetics of β,β-carotene and α-carotene conversion by gNinaB. Product production is displayed as function of substrate concentration. Each reaction was run with 6 μg gNinab (crude extract) for 20 minutes at 28 °C. Curve fitting takes into account the equations of Henderson (39) and Lee and Wilson (40). Values given are the mean ± SD of three independent experiments.
Drosophila NinaB Possesses, Besides Carotenoid-Oxygenase, Intrinsic Isomerase Activity.
Based on our findings with gNinaB, we speculated that Drosophila NinaB possesses, besides carotenoid cleavage, intrinsic isomerase activity. To test this hypothesis, we expressed the enzyme in an E. coli strain, genetically engineered to produce β,β-carotene (2). Because 11-cis-retinal can rapidly undergo light-induced and thermal isomerization, we expressed NinaB at low growth temperature in darkness. With short induction times, significant amounts of 11-cis-retinal became detectable (Fig. 4A, 4B). In contrast, no 11-cis-retinal was found when we expressed a murine β,β-carotene-15,15′-oxgenase in this E. coli strain (Fig. 4A). Thus, Drosophila NinaB, like Galleria NinaB, can produce the 11-cis-retinoid stereoisomer from carotenoids.
Fig. 4.
β,β-carotene and 11-cis-retinal promote rhodopsin production independently of light conditions. (A) Drosophila NinaB was expressed in a β,β-carotene accumulating E. coli strain in the dark at 20 °C to reduce spontaneous thermal isomerization of retinoids. HPLC traces show absorbance at 350 nm from lipid extracts of bacterial pellets. Lower trace was obtained before induction of ninaB expression; middle trace was obtained after 4 hours of induction of NinaB expression; upper trace was obtained after 4 hours of induction of murine CMO1 expression in this bacteria strain. 1, 11-cis-retinaloxime (syn); 2, all-trans-retinaloxime (syn); 3, 13-cis-retinaloxime (syn/anti); 4, 11-cis-retinaloxime (anti); 5, all-trans-retinaloxime (anti); 6, all-trans-retinol. Insets show spectra of peaks 1 and 2 of the middle trace. (B) Kinetics of the conversion of β,β-carotene by Drosophila NinaB. Experimental setup was as described in (A). (C) Immunoblot analysis for the determination of the content of mature Rh1 in Drosophila imago heads after supplementation with β,β-carotene, all-trans-retinal, and 11-cis-retinal, respectively. Controls were ninaE17ol flies (opsin-deficient) and wtB flies (reared on corn medium). Each supplementation group was maintained either in darkness (D) or under a regimen of 16 hours light/8 hours dark (L). Nitrocellulose membranes were reprobed with anti-tubulin antibody as loading control. Molecular weight markers (in kDa) are indicated on the left.
The In Vivo Role of NinaB.
In Drosophila, 11-cis-3-hydroxy-retinal is essential for the production of the visual pigment of rhabdomers R1–6 (Rh1) and its targeting to rhabdomer membranes (19). Carotenoid-deprived flies raised in the dark remain blind even when supplemented with all-trans-retinal (20). Based on our in vitro findings, we speculated that NinaB and carotenoids can light-independently promote Rh1 production. Indeed, wild-type flies produced 11-cis-3-hydroxy-retinal when raised from carotenoids in the dark, whereas ninaB mutants accumulated the carotenoid precursor (Fig. S7). To provide further in vivo evidence for this assumption, we supplemented carotenoid and retinoid-deprived flies, with all-trans-retinal or β,β-carotene. Flies were then kept either in darkness or under light. After 48 hours, we measured levels of mature Rh1 by immunoblot analysis (Fig. 4C). Mature Rh1 has a molecular mass of 32 kDa, indicative for the posttranslational processing of the nascent apo-Rh1 to the mature Rh1 form and its translocation to rhabdomer membranes (19). As shown in Fig. 4C, β,β-carotene promoted Rh1 maturation light-independently, whereas on all-trans-retinal supplementation mature Rh1 was found only in illuminated flies. To directly demonstrate that the 11-cis stereoisomer is required for Rh1 maturation, we supplemented flies with 11-cis-retinal. Indeed, immunoblot analysis demonstrated that 11-cis-retinal like β,β-carotene promoted light-independent Rh1 production (Fig. 4C).
Discussion
We cloned and functionally characterized the ninaB gene product from Galleria mellonella. We show that gNinaB catalyzed an oxidative cleavage at the C15,C15′ double of various carotenoid substrates and an all-trans to 11-cis isomerization of one cleavage product. Using Drosophila as an animal model, we show that this enzymatic property of NinaB is more generally found in insects and promotes light-independent visual pigment production. Thus, we establish a functional link between carotenoid oxygenases and retinoid isomerases by showing that both activities are combined in a single protein in insects.
gNinaB Catyalyzes Oxidative Cleavage and Site-Specific All-trans to 11-cis Isomerization of Carotenoids.
We found that gNinaB efficiently converted various symmetric and asymmetric carotenoids into retinoid derivatives. The observation that only one product was isomerized from the all-trans to 11-cis-configuration indicated that gNinaB specifically interacts with one half site of the substrate. This conclusion was further supported by kinetic analysis. At low substrate concentration, the turnover rate for symmetrical β,β-carotene was twice that of an asymmetric carotenoid such as α-carotene, with one β-ionone and one ε-ionone ring. Moreover, this substrate binding characteristic of gNinaB was also obvious by the isomerization pattern of products from asymmetric carotenoids. Among enzymatic processing of α-carotene and lutein exclusively, the retinoid with the β-ionone ring was found as an 11-cis isomer. Because the ratio of the 11-cis- versus the all-trans-isomer is approximately the same as in symmetrical carotenoids, we conclude that only one half site of the carotenoid substrate is isomerized, but with a very high efficiency.
Because we found that both β,β-carotene and its 3,3′-dihydroxy derivative zeaxanthin were cleaved and isomerized by gNinaB, the hybrid compound cryptoxanthin was of particular interest. Our analysis revealed that gNinaB preferentially isomerized the binding site with the hydroxylated β-ionone ring. With cryptoxanthin, it also is possible to calculate the pertinent enzymatic parameters (kcat/Km) for different carotenoid substrates by determining the relative rates for the corresponding symmetrical substrates in a single experiment (see SI Text). At low substrate concentrations, prevailing in carotenoid metabolism, for a given substrate and enzyme concentration, the reaction velocity essentially depends on this term.
Translating Enzymatic Properties into Structural Predictions.
Recently, a bacterial apo-carotenoid oxygenase has been biochemically and structurally characterized (17, 21). One of its most prominent structural feature is a large kinked tunnel spanning the protein and passing the active center defined by the ferrous iron. This enzyme cleaves apo-β-carotenals, (i.e., carotenoids with a single β-ring) at the 15,15′ double bond and acyclic apo-lycopenals at multiple positions (21). Carotenoids with two β-ionone rings such as β,β carotene are not cleaved because a β-ionon ring cannot pass the narrow tunnel entrance. The selectivity of animal carotene oxygenases, NinaB and vertebrate CMO1, clearly indicate a wider tunnel, as β-ionone rings must enter in a directed orientation. Moreover, gNinaB even accepted β-ring substitutions such as 3-hydroxylation.
Based on structural analysis of the bacterial oxygenase (17), it was suggested that, on binding, the apo-carotenoid substrate is cranked into a cis-trans-cis configuration; however, the quality of the crystallographic data does not firmly establish this mechanism. Moreover, in tests for enzymatic activity all-trans-retinal was the major product and only trace amounts of 13-cis-retinal became detectable (21). Whereas animal visual pigments solely bind cis-retinoid stereoisomers, cyanobacterial opsins readily bind all-trans-retinal to form the bipartite sensory rhodopsin complex (22).
In contrast, our analysis demonstrated that gNinaB efficiently isomerized one half site of the substrate and that this activity is required for visual pigment production. Modifying the structure protein scheme published by Kloer and Schulz (23), we propose a spatial arrangement between substrate (e.g., cryptoxanthin) and NinaB as shown in Fig. S8. This scheme may (apart from the 3-hydoxy-group) be applicable also to vertebrate CMOs. Even though we found no experimental evidence that gNinaB can convert all-trans-retinyl esters (data not shown), this model may also approximately describe the binding of these compounds to RPE65.
Cleavage and Isomerization, Sum to a Negative ΔG °′.
ΔG°′ for the oxidative cleavage of carbon double bond, taking fumaric acid vs. two molecules of glyoxylic acid, has been determined to be −79.3 kcal/mol (24). The free energy consumption for an all-trans to 11-cis isomerization of retinal is 4.1 kcal/mol (25). Thus, in gNinaB the all-trans to 11-cis isomerization can be easily driven by the oxidative cleavage of carotenoids. The surplus energy is surprisingly high, exceeding even the activation energy of +45 kcal/mol (26), for photoisomerization of the chromophore of native rhodopsin. In contrast, the negative free energy of retinyl ester hydrolysis providing energy to drive the endothermic isomerization by RPE65 is only slightly greater than 4.1 kcal/mol (27). As RPE65 is classified as isomerohydrolase, gNinaB should be classified as isomerooxygenase.
Implications for the Insect Visual System.
Moths and butterflies (Lepidoptera), like higher flies (Cyclorrhapha), use 3-hydroxyretinal (A3) as visual chromophore. However, Lepidoptera have a less complicated carotenoid metabolism as compared with higher flies. Whereas Lepidoptera, like other A3-insect groups, use the 3R enantiomer, Drosophila exclusively use the 3S enantiomer of 3-hydrox-retinal as visual chromophore (28, 29). As we show here, the moth NinaB can produce in a one step reaction the visual chromophore from plant-derived zeaxanthin (3,3′-R,R-dihydroxy-β,β-carotene). In higher flies, however, visual chromophore production requires further modifications of the primary cleavage product such R to S enatiomerization and/or hydroxylation reactions (30).
In flies, the 11-cis-retinoid stereoisomer is of importance not only for phototransduction but also for the targeting of rhodopsin to rhabdomer membranes via the secretory pathway (19). This targeting of Rh1 is essential for visual phototransduction but also for photoreceptor development (31). During the latter process, Rh1 plays an important role for the formation of a rhabdomere terminal web (RTW), an actin-based cytoskeletal scaffold (32). A direct involvement of NinaB for Rh1 maturation is supported by our in vivo analysis. We show that β,β-carotene, like 11-cis-retinal, promoted Rh1 maturation in photoreceptors light-independently. In β,β-carotene accumulating E. coli-expressing NinaB, with short induction times in the dark, significant amounts of 11-cis-retinal became detectable. This unique property of NinaB in this process was masked by thermal instability of 11-cis-retinal in our previous study (2). Thus, visual chromophore production from carotenoids via NinaB ensures proper photoreceptor development and function under all ambient light conditions. Since NinaB catalyzed all-trans to 11-cis isomerization of only one half site of the carotenoid substrate, it is likely that the second cleavage product is light-dependently isomerized into the visual chromophore. The existence of such a light-dependent pathway is supported by the observation that all-trans-retinal promoted Rh1 production in light-treated flies (19).
In insects, carotenoids and NinaB are solely required for visual chromophore production (3). Once linked to the opsin moiety, insect visual pigments are usually thermostabile. In vertebrates, oxygenase and isomerase activity are separated into two distinct proteins, CMO1 and RPE65. Indeed, no 11-cis-retinal production was observed when we expressed murine CMO1 under the same conditions as NinaB. A separation of oxygenase and isomerase activity into two distinct proteins, CMO1 and RPE65, might be the consequence of two characteristics of vertebrate biology. First, vertebrate rhodopsin decays after a bleach into the opsin and the all-trans-photoproduct, which must be recycled back into the visual chromophore. This process requires a specific retinoid isomerase. Second, in vertebrates, carotenoid derivatives are also important for gene regulation in the form of the all-trans-stereoisomer of retinoic acid.
In summary, our findings provide a functional link between vertebrate RPE65 and insect NinaB, which are both essential for the synthesis of the visual chromophore (3, 13). The double function of NinaB supports the conclusion that RPE65 is a retinoid isomerase, as it is plausible that members of a family have retained a potential that already had emerged in an ancestor. In addition, the topological relation between enzyme and substrate should be similar in NinaB and RPE65 but also in vertebrate carotenoid oxygenases. Whereas NinaB can be clearly assigned as carotenoid isomerooxygenase, the role of iron and oxygen in the RPE65-dependent isomerohydrolase reaction remains elusive. A comparison between RPE65 and NinaB may allow the prediction of functional site residues that participates in the isomerization and/or oxidative cleavage reaction. It will be fascinating to study the evolution and diversification of this protein family in animals, following the trace from a single multifunctional ancestral protein to several highly specialized enzymes in vertebrates.
Experimental Procedures
Animals.
The large wax moth originated from an infested local bee yard. For breeding, afflicted honey bee combs were placed in air-conditioned containers at 28 °C in darkness. Three different development stages of Galleria mellonella (larvae, pupae, imagines) were collected and frozen at −80 °C until further use.
The wild-type Berlin (WTB) Drosophila melanogaster strain was reared on modified standard corn medium. In this medium corn meal was replaced by carotenoid-free rice meal. Flies were reared for several generations on this medium at 25 °C in a regimen of 16 hours light/8 hours dark. Carotenoid and retinoid deficiency of the flies was verified by immunoblotting for Rh1 and HPLC analysis for carotenoids and retinoids, as previously described (33). To determine Rh1 maturation under different light conditions and supplementation regimens for chromophore precursors, freshly eclosed flies were transferred to media containing 5 μmol/l β-carotene, 100 μmol/l all-trans-retinal, or 11-cis-retinal, respectively. Flies were then kept under a regimen of 16 hours light/8 hours dark or in darkness.
Carotenoids and Retinoids.
α-Carotene [(6′R)-β,ε-carotene] and cryptoxanthin [(3R)-β,β-carotene-3-ol] were purchased from Carotene Nature (Switzerland). β,β-carotene, lutein [(3R,3′R,6′R)-β,ε-carotene-3,3′-diol], and zeaxanthin [(3R,3R′)-β,β-carotene-diol] were purchased from Wild (Germany), and all-trans-retinal, 13-cis-retinal and all-transretinyl esters were purchased from Sigma-Aldrich. 11-cis-Retinal was isolated from dark-adapted bovine eyes by a published method (34). An authentic 11-cis-retinal standard was a kind gift from Dr. K. Palczewski (Case Western Reserve University, Cleveland, OH). 3-Hydroxy-retinal was a kind gift from Drs. H. Mayer and A. Rüttimann (Roche, Basel, Schwitzerland). 3-Hydroxy-retinal oxime stereoismers were identified on basis of published data (35). α-Retinal-oxime and 3-hydroxy-α-retinal-oxime were identified on basis of spectral characteristics (36, 37) and assigned as all-trans-stereoisomers. Carotenoids were purified on high-performance thin layer chromatography plates (Merck). In addition, β,β-carotene was analyzed by reverse-phase HPLC without detecting any trace of the 11-cis isomer. For quantification of the molar amounts of reaction products, peak integrals were scaled with defined amounts of reference substances. Due to a lack of an authentic standard, we used peak integrals for all-trans-retinal and 3-hydroxy-all-transretinal for quantification of the amounts of all-trans-α-retinal and all-trans-3-hydroxy-α-retinal, respectively.
Primers.
Galleria up: 5′-TTC TTC TAC CTG CAC ATC ATC A-3′ Galleria down: 5′-TCC ACT TTA ATT ATT GTT CCA GG-3′ Galleria full up: 5′-ATG GCG GTG CAT CAA GAA AAA CTC TAT CCC AAC-3′ Galleria full down: 5′- CAA CTG GGT GCG ACG AGG TAA GAA CC-3′.
Preparation of Total RNA from Galleria mellonella.
For RNA preparation, the heads of the late pupae were dissected by hand. Total RNA was isolated by the TRIzol method as previously described (33). For further analysis, total RNA was purified using the RNeasy-minikit (Quiagen, Hilden, Germany).
Cloning of cDNAs Encoding gNinaB.
Reverse transcription was carried out using 500 ng of total RNA purified from pupal heads, using an oligo(dT) primer and SuperScript reverse transcriptase (Invitrogen). RNA integrity and origin was determined by RT-PCR for Galleria-Opsin (NCBI Protein-ID AAL59876.1) using the primer Galleria opsin-up and opsin-down (data not shown). A partial Galleria ninaB was amplified with primers galleria-up and galleria-down. For cloning of full-length ninaB cDNA, RACE-PCRs were performed using a 5′/3′ RACE kit (First Choice RLM-RACE Kit, Applied Biosystems). The PCR products were cloned into the vector pCRII TOPO and verified by sequencing. Finally, a full-length cDNA was amplified and ligated into the vector pBAD-TOPO (Invitrogen), resulting in the plasmid pGallox-I.
gNinaB Expression in E. coli and Preparation for In Vitro Enzyme Assay.
For heterologous expression of gNinaB, pGallox-I was transformed in the E. coli strain XL1-blue (Stratagene Inc., La Jolla, CA). Bacteria were grown at 28 °C until an OD600 of 0.5. Protein expression was induced with l-arabinose (0.02% wt/vol) for 8 hours. Bacteria were harvested by centrifugation and broken with a French press in a buffer containing 50 mmol/l Tricine/KOH (pH 7.6, 4 °C), 100 mmol/l NaCl, and protease inhibitor mixture (Roche). The crude protein extract was centrifuged at 20,000 g for 10 minutes at 4 °C. The supernatant was then centrifuged at 100,000 g for 1.5 hours at 4 °C. The resulting supernatant was used for in vitro tests for enzymatic activity or for protein purification by affinity chromatography (Protino Ni 150, Macherey-Nagel). Purity of gNinaB was confirmed by SDS/PAGE and silver staining with the SilverStain Plus kit (Roth).
Expression of NinaB and Murine CMO1 in β,β-Carotene-Expressing E. coli.
An E. coli strain harboring an expression vector containing the set of genes for β,β-carotene production was transformed with expression vectors for NinaB or CMO1 as previously described (2, 8). Bacteria were grown at room temperature in the dark until an OD600 of 0.6. Protein expression was induced by the addition of l-arabinose (final concentration 0.02%). At given time points, volumes containing an equivalent of 10 OD600 bacteria were subjected to HPLC analysis, as previously described (2).
Immunoblotting.
For determination of gNinaB amounts, crude protein extracts were subjected to SDS/PAGE and were electrotransferred onto nitrocellulose membranes (Roth). gNinaB was detected by primary anti-His antibodies (Quiagen). Quantification was carried by the Quantity-one (version 4.6) software (BioRad) in comparison to know amounts of purified gNinaB. Immunoblot analyses of protein extracts of Drosophila heads were carried out as described before (38), using mouse monoclonal anti-Rh1 primary antibody (4C5, Hybridoma Bank). Tubulin was detected using mouse monoclonal β-tubulin antibody (Chemikon MAB3408).
In vitro Enzyme Assays.
For tests for enzymatic activity, we used the method described by (4) with the following modifications. Reactions were run in 2-ml Eppendorf tubes in a volume of 100 μl in 50 mmol/l Tricine/KOH (pH 7.6) with 1% or 3% (wt/vol) OTG. The loading capacity of OTG micelles for β,β-carotene was determined, as described before (33), as being linear up to 16 μmol/l (1% OTG) and 20 μmol/l (3% OTG), respectively. Carotenoids were dissolved in n-hexane (β,β- and α-carotene) or ethanol (all other carotenoids), mixed with OTG and dried in a Speedvac (Eppendorf Concentrator 5301). Protein extract was added under vigorously vortexing for 30 seconds. The reaction mixture was incubated on an Eppendorf shaker (300 rpm, 28 °C) and was stopped by adding 200 μl 2 mol/l hydroxylamine (pH 6.8) and 200 μl methanol. Retinoids and carotenoids were extracted and subjected to HPLC analysis as previously described (2, 33). All steps were carried out under a red dim safety light.
Evaluation of Kinetic Data.
To obtain sufficient product we were forced to incubate for a longer time (20 minutes) with a high enzyme concentration (0.71 μmol/l), comparable to the substrate concentrations. The high enzyme quantity leads to an overestimation of free substrate concentration in case of a strong binding, and the long incubation time may lead to a major underestimation of the initial velocity because a significant part of the substrate is converted. To account for these shortcomings, we implemented the equation of Henderson (39) covering high enzyme concentrations, as well as the equation of Lee and Wilson (40) dealing with substrate turnover, in GOSA software (Bio-Log) to fit the kinetic parameters. As the turnover number was extremely small (≤0.5/min), the rapid equilibrium approach, with Km = KD was adequate.
Supplementary Material
Acknowledgments.
The authors thank Drs. H. Mayer and A. Rüttimann for the gift of 3-hydrox-retinal. We are grateful to Dr. K. Palzcewski for valuable discussions and the gift of 11-cis-retinal. The work was supported by a grant from the Ministry of Science and Art (Baden-Württemberg, Germany) and by start-up funds of the Case Western Reserve University (Cleveland, OH).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. AM778534).
This article contains supporting information online at www.pnas.org/cgi/content/full/0807805105/DCSupplemental.
References
- 1.Palczewski K. G protein-coupled receptor rhodopsin. Annu Rev Biochem. 2006;75:743–767. doi: 10.1146/annurev.biochem.75.103004.142743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 3.von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation invivo. Proc Natl Acad Sci USA. 2001;98:1130–1135. doi: 10.1073/pnas.031576398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J Biol Chem. 2002;277:23942–23948. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 5.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, et al. Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15′-dioxygenase. J Biol Chem. 2001;276:6560–6565. doi: 10.1074/jbc.M009030200. [DOI] [PubMed] [Google Scholar]
- 6.Wyss A, Wirtz G, Woggon W, Brugger R, Wyss M, et al. Cloning and expression of beta,beta-carotene 15,15′-dioxygenase. Biochem Biophys Res Commun. 2000;271:334–336. doi: 10.1006/bbrc.2000.2619. [DOI] [PubMed] [Google Scholar]
- 7.Yan W, Jang GF, Haeseleer F, Esumi N, Chang J, et al. Cloning and characterization of a human beta,beta-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics. 2001;72:193–202. doi: 10.1006/geno.2000.6476. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 9.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, et al. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, et al. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 14.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, et al. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–131. doi: 10.1016/j.bbadis.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 16.Isono K, Tanimura T, Oda Y, Tsukahara Y. Dependency on light and vitamin A derivatives of the biogenesis of 3-hydroxyretinal and visual pigment in the compound eyes of Drosophila melanogaster. J Gen Physiol. 1988;92:587–600. doi: 10.1085/jgp.92.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloer DP, Ruch S, Al-Babili S, Beyer P, Schulz GE. The structure of a retinal-forming carotenoid oxygenase. Science. 2005;308:267–269. doi: 10.1126/science.1108965. [DOI] [PubMed] [Google Scholar]
- 18.Seki T, Vogt K. Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Comp Biochem Physiol. 1998;119B:53264. [Google Scholar]
- 19.Ozaki K, Nagatani H, Ozaki M, Tokunaga F. Maturation of major Drosophila rhodopsin, NinaE, requires chromophore 3-hydroxyretinal. Neuron. 1993;10:1113–1119. doi: 10.1016/0896-6273(93)90059-z. [DOI] [PubMed] [Google Scholar]
- 20.Seki T, Fujishita S, Ito M, Matsuoka N, Kobayashi C, et al. A fly, Drosophila melanogaster, forms 11-cis 3-hydroxyretinal in the dark. Vision Res. 1986;26:255–258. doi: 10.1016/0042-6989(86)90020-9. [DOI] [PubMed] [Google Scholar]
- 21.Ruch S, Beyer P, Ernst H, Al-Babili S. Retinal biosynthesis in Eubacteria: In vitro characterization of a novel carotenoid oxygenase from Synechocystis sp. PCC 6803. Mol Microbiol. 2005;55:1015–1024. doi: 10.1111/j.1365-2958.2004.04460.x. [DOI] [PubMed] [Google Scholar]
- 22.Jung KH, Trivedi VD, Spudich JL. Demonstration of a sensory rhodopsin in eubacteria. Mol Microbiol. 2003;47:1513–1522. doi: 10.1046/j.1365-2958.2003.03395.x. [DOI] [PubMed] [Google Scholar]
- 23.Kloer DP, Schulz GE. Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci. 2006;63:2291–2303. doi: 10.1007/s00018-006-6176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thauer RK, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rando RR, Chang A. Studies on the catalyzed interconversions of vitamin A derivatives. J Am Chem Soc. 1983;105:2879–2882. [Google Scholar]
- 26.Ala-Laurila P, Donner K, Koskelainen A. Thermal activation and photoactivation of visual pigments. Biophys J. 2004;86:3653–3662. doi: 10.1529/biophysj.103.035626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernstein PS, Law WC, Rando RR. Biochemical characterization of the retinoid isomerase system of the eye. J Biol Chem. 1987;262:16848–16857. [PubMed] [Google Scholar]
- 28.Seki T, Isono K, Ito M, Katsuta Y. Flies in the group Cyclorrhapha use (3S)-3-hydroxyretinal as a unique visual pigment chromophore. Eur J Biochem. 1994;226:691–696. doi: 10.1111/j.1432-1033.1994.tb20097.x. [DOI] [PubMed] [Google Scholar]
- 29.Seki T, Vogt K. Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Compar Biochem Physiol. 1998;119B:53–64. [Google Scholar]
- 30.Seki T, Isono K, Ozaki K, Tsukahara Y, Shibata-Katsuta Y, et al. The metabolic pathway of visual pigment chromophore formation in Drosophila melanogaster—all-trans (3S)-3-hydroxyretinal is formed from all-trans retinal via (3R)-3-hydroxyretinal in the dark. Eur J Biochem. 1998;257:522–527. doi: 10.1046/j.1432-1327.1998.2570522.x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar JP, Ready DF. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 32.Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- 33.Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, et al. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–13437. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- 34.Gartner W, Ullrich D, Vogt K. Quantum yield of CHAPSO-solubilized rhodopsin and 3-hydroxy retinal containing bovine opsin. Photochem Photobiol. 1991;54:1047–1055. doi: 10.1111/j.1751-1097.1991.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 35.Gartner W, Plangger A. 3-Hydroxy retinal, a new chromophore identified in insect eyes: HPLC separation and NMR spectroscopic identification of oxim forms. Z Naturforsch. 1988;43c:473–475. [Google Scholar]
- 36.Yajie Wang, Lugtenburg J. 4,5-Didehydro-9-demethyl-9-halo-5,6-dihydroretinals and their 9-cyclopropyl and 9-isopropyl derivatives-simple preparation of alpha-ionone derivatives and pure (all-E)-, (9Z)- and (11Z)-alpha-retinals. Eur J Organ Chem. 2004;2004:3497–3510. [Google Scholar]
- 37.Wang Y, Bovee-Geurts PH, Lugtenburg J, DeGrip WJ. Alpha-retinals as rhodopsin chromophores-preference for the 9-Z configuration and partial agonist activity. Photochem Photobiol. 2008;84:889–894. doi: 10.1111/j.1751-1097.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiefer C, Sumser E, Wernet MF, von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci USA. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson PJ. Steady-state enzyme kinetics with high-affinity substrates or inhibitors. A statistical treatment of dose-response curves. Biochem J. 1973;135:101–107. doi: 10.1042/bj1350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HJ, Wilson IB. Enzymic parameters: Measurement of V and Km. Biochim Biophys Acta. 1971;242:519–522. doi: 10.1016/0005-2744(71)90144-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.