Abstract
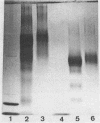
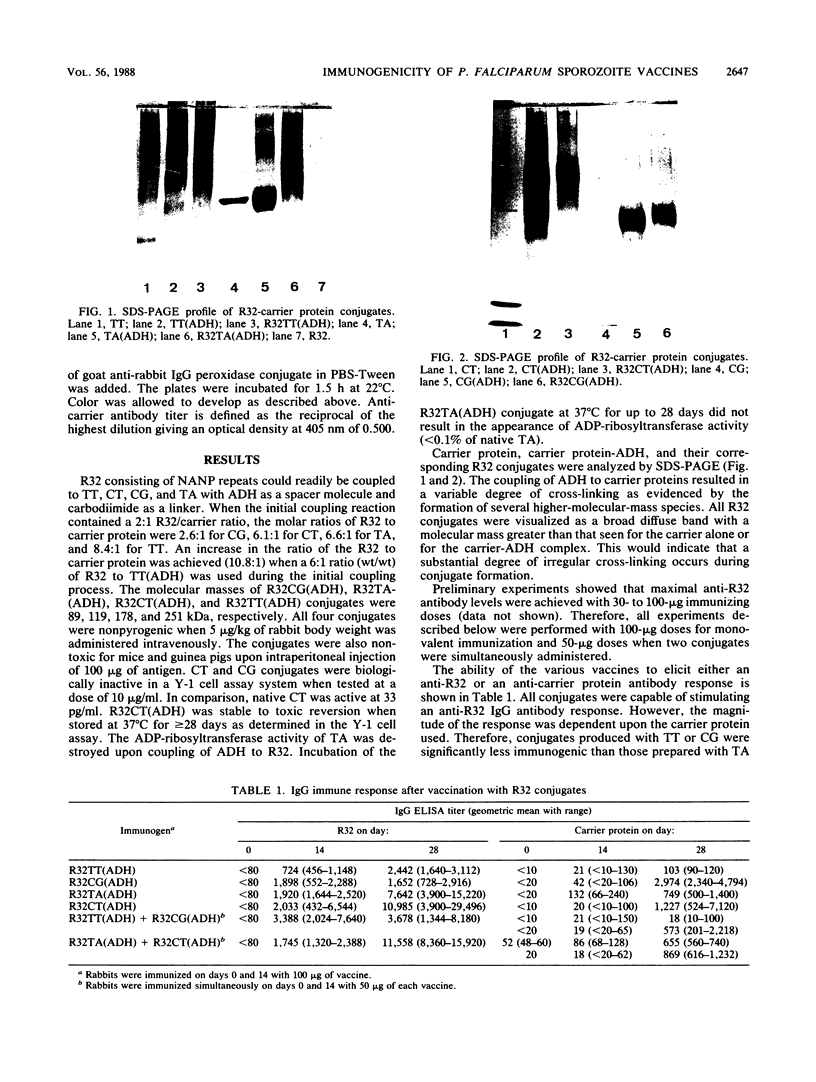
Conjugate vaccines against the sporozoite stage of Plasmodium falciparum were synthesized by covalently coupling the recombinant protein R32 [with the one-letter amino acid code of MDP-[(NANP)15NVDP]2LR] to tetanus toxoid, cholera toxin, choleragenoid, and Pseudomonas aeruginosa toxin A. Conjugates were produced by using adipic acid dihydrazide as a spacer molecule and carbodiimide as a coupling agent. The molar ratio of R32 to carrier protein ranged from 2.5:1 to 8.4:1. These conjugates were found to be stable, nontoxic, and nonpyrogenic. When adsorbed onto Al(OH)3, all conjugates were capable of inducing anti-R32 antibody. Conjugates made with either cholera toxin or Pseudomonas aeruginosa toxin A were significantly more immunogenic than those constructed with tetanus toxoid or choleragenoid. However, the magnitude of the immune response to the R32 moiety was not governed by the antibody response to the carrier protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Cross A. S., Wegmann A., Germanier R., Sadoff J. C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Invest. 1987 Jul;80(1):51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Lang A. B., Sadoff J. C., Germanier R., Fürer E. Vaccine potential of Pseudomonas aeruginosa O-polysaccharide-toxin A conjugates. Infect Immun. 1987 Jul;55(7):1547–1551. doi: 10.1128/iai.55.7.1547-1551.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Sadoff J. C., Fürer E., Germanier R. Pseudomonas aeruginosa polysaccharide-tetanus toxoid conjugate vaccine: safety and immunogenicity in humans. J Infect Dis. 1986 Oct;154(4):682–688. doi: 10.1093/infdis/154.4.682. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Cannon L. T., Sr, Berzofsky J. A., Majarian W. R., Young J. F., Maloy W. L., Hockmeyer W. T. Plasmodium falciparum: sporozoite boosting of immunity due to a T-cell epitope on a sporozoite vaccine. Exp Parasitol. 1987 Aug;64(1):64–70. doi: 10.1016/0014-4894(87)90009-9. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Takai E., Ohnuma H., Kitajima K., Tsuda F., Machida A., Mishiro S., Nakamura T., Miyakawa Y., Mayumi M. A synthetic peptide vaccine involving the product of the pre-S(2) region of hepatitis B virus DNA: protective efficacy in chimpanzees. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9174–9178. doi: 10.1073/pnas.83.23.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lise L. D., Mazier D., Jolivet M., Audibert F., Chedid L., Schlesinger D. Enhanced epitopic response to a synthetic human malarial peptide by preimmunization with tetanus toxoid carrier. Infect Immun. 1987 Nov;55(11):2658–2661. doi: 10.1128/iai.55.11.2658-2661.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig R. S., Nussenzweig V. Development of sporozoite vaccines. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):117–128. doi: 10.1098/rstb.1984.0113. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze M. P., Leclerc C., Jolivet M., Audibert F., Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985 Oct;135(4):2319–2322. [PubMed] [Google Scholar]
- Simonsen O., Klaerke M., Klaerke A., Bloch A. V., Hansen B. R., Hald N., Hau C., Heron I. Revaccination of adults against diphtheria. II: Combined diphtheria and tetanus revaccination with different doses of diphtheria toxoid 20 years after primary vaccination. Acta Pathol Microbiol Immunol Scand C. 1986 Oct;94(5):219–225. doi: 10.1111/j.1699-0463.1986.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Nussenzweig R. S., Potocnjak P., Nussenzweig V., Aikawa M. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science. 1980 Jan 4;207(4426):71–73. doi: 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- Young J. F., Ballou W. R., Hockmeyer W. T. Developing a human malaria sporozoite vaccine. Microb Pathog. 1987 Apr;2(4):237–240. doi: 10.1016/0882-4010(87)90121-5. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]