There is now little doubt that the prime cause of the motor-neuron disease amyotrophic lateral sclerosis (ALS) is related to misfolding and aggregation of the metalloenzyme Cu/Zn superoxide dismutase (SOD1). Yet, SOD1 has turned out remarkably resistant to aggregation under physiological conditions in vitro. Pathologic mutant proteins that are destabilized to the extent that they fail to fold can still be stored at room temperature for long periods of time without notable signs of self-association. To produce SOD1 aggregates one has to resort to more radical measures, such as high temperature at low pH (1, 2), organic solvents (1), or oxidative cross-linking of the protein's solvent-accessible cysteines (3). Because these conditions are different from those in the cell the connection between SOD1 aggregation and ALS has remained elusive; after all, most if not all proteins contain sequences capable of nucleating aggregation (4) and can thus be forced to aggregate in vitro if the conditions are made sufficiently “persuasive.” In this issue of PNAS, Valentine and collaborators (5) present a new interesting piece to the puzzle by showing that SOD1 can be made to aggregate under conditions that are physiologically relevant. The trick seems to be simply that the solution requires appropriate agitation. After a few hours of this treatment in an orbital shaker at 250 rpm, immature molecules of wild-type SOD1 nucleate into ordered fibrillar structures similar to those observed in classical amyloidoses such as Alzheimer's and Huntington's diseases. For nucleation to occur, however, the protein also needs to be demetallated and without its stabilizing disulfide bridge. Once the seeds are formed the process can recruit more stable SOD1 species for the fibril elongation. Similar results were published independently by Furukawa et al. (6) just a few months ago.
The question then arises, which part of the SOD1 structure promotes the fibrillation? For disordered proteins like the Aβ peptide associated with Alzheimer's disease, the aggregation propensity is simply determined by the local sequence signatures that are constantly accessible to the solvent (7). In the case of SOD1, such sequence signatures could be hidden inside the folded structure (F) and exposed for aggregation only in the unfolded state (U) (Fig. 1). The level of aggregation-competent material becomes then controlled by protein stability, i.e., ΔG = −RT ln[F]/[U]. The less stable the protein is, the higher is the fraction of reactive molecules. Consistent with this model, the ALS-provoking SOD1 mutations are found to decrease protein stability and/or the protein's net repulsive charge (8, 9). This seems to be a typical feature for the aggregation of proteins: the exposure of the sticky sequence through loss of stability promote aggregation nucleation and the repulsive charges help to reduce the rate of aggregation (10). Even so, it is not directly obvious which part of the protein that causes the problem because the SOD1 sequence seems completely devoid of the “sticky” hydrophobic patches that characterize most other globular proteins. A simple way to convince oneself about this peculiarity is to run SOD1 through one of the predictors for protein aggregation, e.g., TANGO (http://tango.crg.es/). It is conceivable that the overall hydrophilic nature of the SOD1 sequence is required for maintaining the immature states of the protein soluble inside the cell. Nevertheless, SOD1 displays two distinct sequence segments with intrinsic propensities for fibril formation as determined by the WALTZ algorithm (http://switpc7.vub.ac.be/cgi-bin/submit.cgi), one in the center of the major β-sheet (β2) and one at its edge (β6). This second level of aggregation signals emphasizes that protein aggregation is not a uniform phenomenon but encompasses considerable diversity at the level of both macroscopic morphology and mechanism of formation. At one extreme is the formation of amorphous precipitates that is typically driven by sequence hydrophobicity, and at the other end of the scale is the nucleated and highly ordered assembly of fibrils that can also involve more hydrophilic sequence signatures (11). It will be interesting to see whether these predictions at local sequence level capture also the fibrillation mechanism of the full-length protein.
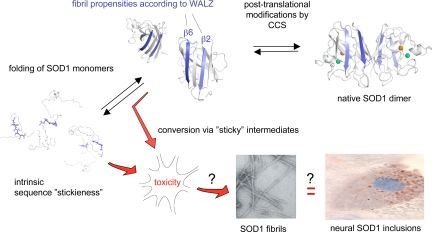
Fig. 1.
Hypothetical outline of the folding and aggregation pathways of SOD1 and how these can be linked to neurotoxicity in ALS. CCS, copper chaperone for SOD1. The EM image of the SOD1 fibrils is adapted from the article of Chattopadhyay et al. (5) in this issue of PNAS, and the fibrillar propensities of the SOD1 sequence as predicted by WALZ are shown in blue. It remains now to find out whether SOD1 fibrillation is linked to neurotoxicity and deposition of proteinaceous inclusions, along the same lines as have been implicated for other neurodegenerative diseases.
We are then left with the big question, what is the coupling between the SOD1 fibrillation observed by Valentine and collaborators (5) and ALS? The identity of the SOD1 species that causes neural damage is still controversial. One line of evidence suggests that the cytotoxic properties stem from erroneous cross-linking of the free cysteines C6 and C111. In favor of this idea, metal-free SOD1 forms soluble disulfide-linked oligomers that are stable over long time scales in vitro. Also, the susceptibility of the protein to undergo such oligomerization is promoted by several ALS-associated SOD1 mutations (3), and the toxicity of SOD1 expressed in cultured cells is lost upon mutational removal of C6 and C111 (12). On the other hand, the degeneration of spinal cord in transgenic mice seem to be caused mainly by apo-SOD1 species with reduced cysteines (13). Some results even challenge the whole thought of aggregation as the primary cause of toxicity by pointing at soluble misfolded protein as the common denominator for several ALS mutations in mice (14). Moreover, incompletely metallated SOD1 can boost neural apoptosis by the gain of detrimental catalytic function (15) and there is evidence that mutant SOD1 also stresses the cells by overloading the chaperone system (16). Adding to these tentative causes of ALS we have now the possibility of pathogenic SOD1 fibrillation (5) along the same lines as postulated for several other neurodegenerative diseases (7, 17). From a reductionist perspective it is attractive to have such a unifying description of the toxicity mechanism. Even so, the connection between protein fibrillation and neural damage still conceals some complexity that needs to be resolved. The most obvious relates to the identity of the pathogenic species. The primary cause of cytotoxicity seems not to be the mature protein fibrils, but rather some much smaller, oligomeric species that accumulate earlier on. In Alzheimer's disease this could be as small as a dimer of Aβ (18). It remains now to find out what could be the corresponding species of SOD1. Also, it is not clear whether the pathogenic species are actually on route to the mature fibrils or if they represent intermediates in competing side reactions (19). In this context it is interesting to note that studies of Aβ aggregation in Caenorhabditis elegans have indicated that cells have the capacity to actively sequester proteins in the form of fibrillar structures (20). The proposed role of such emergency deposition is to buffer transient overload of the degradation system. In the same way as protein sequences have been equipped with specific signatures for degradation and chaperone recognition (10), they would then need sequence signatures for organized deposition. Otherwise the process would escape biological control. This would certainly be an interesting twist to the problem. After all, the propensity to fibrillate is common to a wide range of proteins and peptides, most of which are not associated with protein-misfolding disease. Speculations like these may soon come to test. The finding that SOD1 forms amyloid-like fibrils under physiological conditions (5) will focus attention on this protein as a new promising model system for elucidating the intricacies of neurodegenerative disease. In contrast to most other disease precursors, e.g., Aβ, huntingtin, and the prion protein PrP (7), SOD1 is a globular protein with well-defined structure and folding behavior that easily lends itself to detailed biophysical characterization (8). Moreover, there are more than 120 ALS-associated SOD1 mutations with full disease penetrance that are awaiting closer analysis. The answer to what actually triggers the toxic gain of function is most likely hidden in this material already. It is just a matter of digging it out!
SOD1 can be made to aggregate under conditions that are physiologically relevant.
Acknowledgments.
M.O. thanks the Swedish Research Council, the Knut and Alice Wallenberg Foundation, and Hjärnfonden for financial support.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18663.
References
- 1.Stathopulos PB, et al. Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc Natl Acad Sci USA. 2003;100:7021–7026. doi: 10.1073/pnas.1237797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiDonato M, et al. ALS mutants of human superoxide dismutase form fibrous aggregates via framework destabilization. J Mol Biol. 2003;332:601–615. doi: 10.1016/s0022-2836(03)00889-1. [DOI] [PubMed] [Google Scholar]
- 3.Banci L, et al. SOD1 and amyotrophic lateral sclerosis: Mutations and oligomerization. PLoS ONE. 2008;3:e1677. doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linding R, Schymkowitz J, Rousseau F, Diella F, Serrano L. A comparative study of the relationship between protein structure and beta-aggregation in globular and intrinsically disordered proteins. J Mol Biol. 2004;342:345–353. doi: 10.1016/j.jmb.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay M, et al. Initiation and elongation in fibrillation of ALS-linked superoxide dismutase. Proc Natl Acad Sci USA. 2008;105:18663–18668. doi: 10.1073/pnas.0807058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa Y, Kaneko K, Yamanaka K, O'Halloran TV, Nukina N. Complete loss of post-translational modifications triggers fibrillar aggregation of SOD1 in familial form of amyotrophic lateral sclerosis. J Biol Chem. 2008;283:24167–24176. doi: 10.1074/jbc.M802083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg MJ, Bystrom R, Boknas N, Andersen PM, Oliveberg M. Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proc Natl Acad Sci USA. 2005;102:9754–9759. doi: 10.1073/pnas.0501957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandelin E, Nordlund A, Andersen PM, Marklund SS, Oliveberg M. Amyotrophic lateral sclerosis-associated copper/zinc superoxide dismutase mutations preferentially reduce the repulsive charge of the proteins. J Biol Chem. 2007;282:21230–21236. doi: 10.1074/jbc.M700765200. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau F, Serrano L, Schymkowitz JW. How evolutionary pressure against protein aggregation shaped chaperone specificity. J Mol Biol. 2006;355:1037–1047. doi: 10.1016/j.jmb.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau F, Schymkowitz J, Serrano L. Protein aggregation and amyloidosis: Confusion of the kinds? Curr Opin Struct Biol. 2006;16:118–126. doi: 10.1016/j.sbi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Niwa J, et al. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem. 2007;282:28087–28095. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson PA, et al. Disulfide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 14.Zetterstrom P, et al. Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proc Natl Acad Sci USA. 2007;104:14157–14162. doi: 10.1073/pnas.0700477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estevez AG, et al. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 16.Okado-Matsumoto A, Fridovich I. Amyotrophic lateral sclerosis: A proposed mechanism. Proc Natl Acad Sci USA. 2002;99:9010–9014. doi: 10.1073/pnas.132260399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh DM, Selkoe DJ. Aβ oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 18.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otzen DE, Oliveberg M. Transient formation of nano-crystalline structures during fibrillation of an Aβ-like peptide. Protein Sci. 2004;13:1417–1421. doi: 10.1110/ps.03538904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]