Abstract
Environmental noise that reduces the probability that animals will detect communicative signals poses a special challenge for long-range communication. The application of signal-detection theory to animal communication lead to the prediction that signals directed at distant receivers in noisy environments will begin with conspicuous “alerting” components to attract the attention of receivers, before delivery of the information-rich portion of the signal. Whether animals actually adopt this strategy is not clear, despite suggestions that alerts might exist in a variety of taxa. By using a combination of behavioral observations and experimental manipulations with robotic lizard “playbacks,” we show that free-living territorial Anolis lizards add an “alert” to visual displays when communicating to distant receivers in situations of poor visibility, and that these introductory alerts in turn enhance signal detection in adverse signaling conditions. Our results show that Anolis lizards are able to evaluate environmental conditions that affect the degradation of long-distance signals and adjust their behavior accordingly. This study demonstrates that free-living animals enhance the efficiency of long-range communication through the modulation of signal design and the facultative addition of an alert. Our findings confirm that alert signals are an important strategy for communicating in “noisy” conditions and suggest a reexamination of the existence of alerts in other animals relying on long-range communication.
Keywords: animal signals, habitat noise, robotic lizard playbacks, tropical lizard
Social communication requires that signals be successively transmitted through the environment and detected by receivers. How animals minimize the effects of environmental degradation on signal propagation is a classic focus of communication research (1–3) and is often central to understanding the way animals communicate (4–6) and interact with each other (7–9). Signal detection theory (10) and empirical studies (3) show that simple signal components suffer lower rates of environmental attenuation than more complex, information-rich components. This results in a fundamental tradeoff in the evolution of signal design. A solution would be the production of a composite signal beginning with a simple conspicuous component (an “alert”) that attracts the attention of a receiver, which is then followed by a more detailed “message” component (11). The strategy may be common; possible examples include the simple introductory notes of territorial calls in many birds (10, 12, 13), initial low frequency components of frog mating calls (14), and barks before howls in coyotes (15). Surprisingly, however, the assumption that putative alerting components facilitate signal detection has rarely been confirmed (11, 16), and the explicit predictions that alert use will be more prevalent and more effective in noisy environments have never been tested.
Male lizards of the genus Anolis advertise territory ownership to neighbors and intruders via dynamic visual displays consisting of species-typical sequences of headbobs and extensions of a colored dewlap. Early investigations into the structure of these displays suggested that some species begin their displays with exaggerated introductory movements to facilitate signal detection against visually noisy backgrounds of windblown vegetation (17) or to improve detection when displays are directed at distant conspecifics (18). We observed that several species of Puerto Rican anole often initiated displays with a series of exaggerated 4-legged pushups before delivering the species-typical headbob sequence. These introductory pushups exhibit the same dynamic visual characteristics of high-speed, abrupt movement that laboratory experiments using artificial flag stimuli suggest might elicit orienting responses from inattentive lizards (19, 20).
If high-speed introductory movements alert inattentive receivers, and if there are any costs to alert production (e.g., energy expenditure or attracting the unwanted attention of predators), then lizards should restrict the use of alerts to conditions in which they are required for signal reception. That is, lizards should produce alerts facultatively, increasing the proportion of displays introduced by exaggerated pushups as viewing conditions deteriorate and as the distance to receivers increases. To date, there have been no attempts to study the facultative use of alerts in animal communication systems even though facultative alert production is implied by signal detection theory (10). Its implications, however, are fundamental in terms of whether animals are able to assess the conditions affecting signal degradation and subsequently change the design of signals to compensate. Studies showing that birds (4) and other animals (21, 22) change the way acoustic signals are delivered in noisy environments, and evidence that frogs alter the design of conspicuous mating calls when predation risk is high (23, 24), infers animals might also tailor the type of components included in a signal as conditions affecting signal detection fluctuate. The discovery of facultative alerts in lizards would also suggest a reevaluation of the importance of putative alerts in animals that communicate acoustically, where alert signals were first predicted (10) but have rarely been examined directly (see ref. 11 for apparently the only experimental investigation of an acoustic alert).
To determine whether lizards use exaggerated introductory pushups as alerts, we conducted extensive observations on the yellow-chinned anole, Anolis gundlachi, in the deep-shade forests of Luquillo Mountains in northeastern Puerto Rico. Adult male A. gundlachi use elevated perches within their territory to perform broadcast displays to an audience of neighboring males and females located at various distances throughout the surrounding habitat. Communication is complicated by periods in which ambient light levels are low or “environmental visual noise” is high, as a result of movement from windblown plants and shifting sunspots around the signaling individual. Both poor light and visual noise are predicted to reduce the distance over which dynamic visual displays are detected (6, 17, 25, 26). We then supplemented our observations with a series of field “playback” experiments using robotic lizards (27) to test whether introductory pushups improve the detectability of broadcast displays under visually challenging conditions. Each robot was programmed to precisely mimic the natural display movements of A. gundlachi, including the 4-legged pushup (the “alert”), species-typical head bob (the “message” component), and extension of dewlaps color-matched to the dewlaps of live animals by using a spectrophotometer. We created one display sequence in which the species-typical headbob was performed without introductory movements (“no alert”), and a second in which the species-typical headbob was preceded by introductory pushups (“alert, typical”). We also created a third artificial display sequence in which the species-typical display began with a series of rapid dewlap extensions (“alert, novel”) to test the hypothesis that any high-speed movement opening a display would function as an effective alert. Rapid dewlap extensions are produced at the beginning of displays in other Anolis (e.g., A. opalinus on Jamaica), but never by A. gundlachi. Each focal lizard in the field was allowed seven minutes to view a stationary robot, after which the robot began to display, and we measured the time when the focal lizard first oriented toward the displaying robot.
Results
Alert Production by Free-living Lizards.
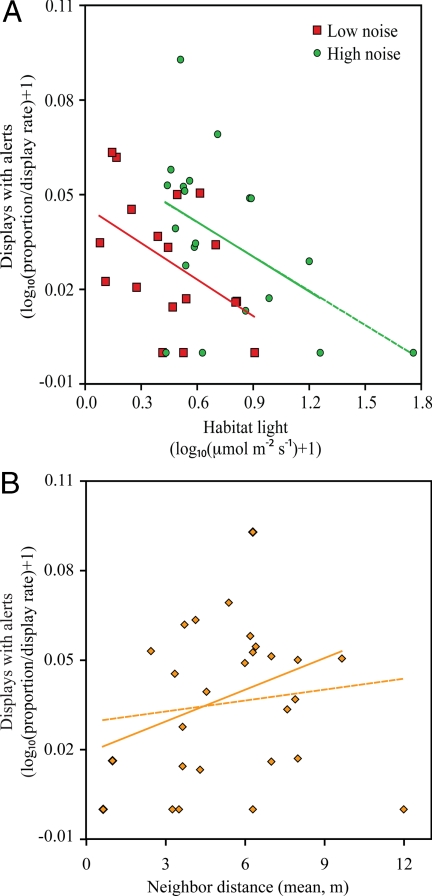
We considered only males with at least one adult male neighbor (26 focal lizards where neighbors here include all male conspecifics within the line-of-sight of a territory holder) and examined the proportion of displays beginning with 4-legged pushups, converted into a weighted index that accounted for overall display production. Our analyses revealed compelling support for introductory pushups used as alerts. Fluctuations in habitat light, visual noise, and the average distance to neighbors predicted the addition of introductory pushups, explaining a total of 59% of the variance in the production of these movements in a multiple regression analysis (Table 1). Specifically, as the distance to receivers increased and viewing conditions deteriorated as a result of low light and high visual noise, lizards produced more broadcast displays that began with exaggerated introductory pushups (Fig. 1; Movie S1 and Movie S2).
Table 1.
Factors predicting the production of displays with introductory “alert” 4-legged pushups by A. gundlachi in a multiple regression.
| Overall regression model (n = 26): r2 = 0.59, F(3,22) = 10.65, P < 0.001 | |||
|---|---|---|---|
| Independent variable | Partial r | t | P |
| Distance to neighbors | 0.40 | 2.07 | 0.050 |
| Visual noise (low vs. high) | 0.63 | 3.78 | 0.001 |
| Habitat light | −0.74 | −5.10 | <0.001 |
All interactions between factors were also considered but did not contribute significantly to the model (P > 0.40) and were sequentially removed.
Fig. 1.
Production of territorial displays with and without “alert” components by A. gundlachi. The proportion of displays by free-living lizards that began with exaggerated 4-legged pushups (alerts) as a function of (A) the amount of habitat light and visual noise experienced at the time of display, and (B) mean distance to male neighbors. The dashed line was calculated with the outlier included. The removal of this outlier resulted in no qualitative changes to our findings. “Noise” refers to the amount of environmental motion occurring in the surrounding habitat. Movie S1 and Movie S2 show wild lizards performing displays with and without introductory pushups.
Alert Detection by Receivers.
Overall, the time to the first orientation response by focal lizards following the onset of the robot display ranged from 0.4 s (during the first movement by the robot) to 207 s (65 seconds after the end of the second display by the robot). The majority of lizards (260 of 331, 79%) oriented toward the displaying robot during the robot”s first display (a response time of ≤25 s) and all but four lizards (99%) oriented toward the robot by the end of its second display (≤142 s).
Playback experiment 1: “Typical-receiver distance”.
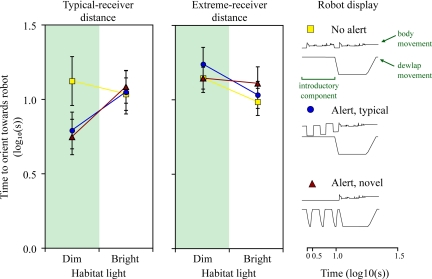
We positioned the robots to approximate the average distance to male neighbors for the lizards in our observational study (Fig. 1B). We predicted that at the distances typical for communication in this species, exaggerated introductory movements would enhance receiver responses under poor viewing conditions. This was indeed what we found; high-speed introductory movements greatly reduced the time required for receivers to attend to a broadcast display under poor light conditions. In poor light, lizards oriented more quickly when the display was preceded by either the species-typical alert (4-legged pushups; “alert, typical”) or by the novel alert (rapid dewlap extensions; “alert, novel”) than when the display lacked any alert (“no alert”; Table 2A, Fig. 2). The equivalently rapid responses to novel and species-typical alerts under low light supports the prediction that alerts attract the attention of the receiver, but do not affect the “message” of a signal (10). In contrast, under bright light, orientation times were comparable for displays with and without alerts. Hence, as predicted, alerts affect receiver responses under challenging conditions, but not under conditions favorable for signal detection.
Table 2.
Factors (F), covariates (C) and their interactions in univariate general linear models found to predict the latency for A. gundlachi to orient towards robot displays given at distances (A) typical of most receivers and (B) extreme for receivers
| A | Typical-receiver distance: overall model, F(10,147) = 3.82, P < 0.001 |
|||
|---|---|---|---|---|
| Independent variable | d.f. | F | P | |
| Intercept | 1 | 5.01 | 0.027 | |
| Display-type (F) | 2 | 0.52 | 0.596 | |
| Light (C) | 1 | 1.32 | 0.253 | |
| Visual noise (C) | 1 | 2.23 | 0.138 | |
| Robot ID (C) | 1 | 0.04 | 0.843 | |
| Display-type*light | 2 | 10.11 | <0.001 | |
| Display-type*light*robot ID | 3 | 7.40 | <0.001 | |
| B | Extreme-receiver distance: overall model, F(7,164) = 2.26, P = 0.032 |
|||
| Independent variable | d.f. | F | P | |
| Intercept | 1 | 39.77 | <0.001 | |
| Display-type (F) | 2 | 4.03 | 0.020 | |
| Light (C) | 1 | 5.62 | 0.019 | |
| Visual noise (C) | 1 | 1.60 | 0.207 | |
| Robot ID (C) | 1 | 0.54 | 0.464 | |
| Display-type*robot ID | 2 | 4.44 | 0.013 | |
All covariate interactions with display-type (F) were initially considered and only those significantly contributing to the final model were retained (P < 0.10).
Fig. 2.
Latency for A. gundlachi to orient toward robot displays with or without exaggerated introductory movements given at distances typical of most receivers and extreme for receivers (typical receiver distances: “no alert” in which the species-typical display is repeated, n = 53; “alert, typical”, 4-legged pushups are placed at the start of the species-typical display, n = 53; “alert, novel” an artificial alert created by the addition of rapid dewlaps to the start of the species-typical display, n = 52; extreme receiver distances: “no alert” n = 59; “alert, typical” n = 57; “alert, novel” n = 56). Plots show mean ± 1 SE orientation times for lizards in poor (“dim”) and bright light conditions (see Figs. S4 and S5). The displays performed by the robot are illustrated as display-action-pattern graphs depicting the movement of the body (upper line) and dewlap (lower line) over time. Robot displays are also shown in Movies S3, Movie S4, and Movie S5.
For displays with alerts, response times were faster under poor light than under bright conditions (Fig. 2). One possible explanation is that lizards monitor conspecifics more closely under poor light, perhaps because it is more difficult for lizards to detect changes in the behavior of previously detected conspecifics under poor light conditions. To test this hypothesis, we compared the number of head movements directed toward still robots to the number of head movements directed at a control object. As one would expect, focal animals turned their heads more often to a stationary robot model than to the control object. More important, our results supported the hypothesis that lizards monitor conspecifics more often under low light conditions. A general linear model revealed a significant interaction between treatment (still robot versus control) and habitat light [ANCOVA: F1,146 = 4.70, P = 0.032; see supporting information (SI) Table S1]. In particular, the difference between the number of head-turns directed at a still robot versus the number of head-turns directed toward the control object increased as light levels declined (Fig. S1). These results support the notion that focal animals in the playback experiment responded faster to the displaying robot under low than high light conditions because focal lizards glance more frequently at conspecifics when light levels are poor.
Playback experiment 2: “Extreme-receiver distance”.
We repeated our experiment with the robots positioned at distances that exceeded the distances to potential recipients in our initial study. Since under natural conditions alerts are not used to communicate with conspecifics over such extreme distances, we predicted that lizards would have difficulty detecting any display (with or without alerts) under low light conditions in this experiment. As predicted, the addition of alerts to displays had no discernable effect on orientation times under low light conditions when the robots were positioned at extreme distances from the focal lizards. In bright light, orientation times were similar to those for the same displays at typical-receiver distances, but as light deteriorated, orientation times for any of the displays became longer (Table 2B; Fig. 2; see also Interaction terms involving robot ID).
Interaction terms involving robot ID.
In presentations for both typical-receiver and extreme-receiver distances (experiments 1 and 2, respectively), significant interactions were found with robot ID (corresponding to the three different robot models used in the experiments). We could find no obvious differences between the robots other than the general morphology of models (the same display programs were used for all robots; the construction of the robots were identical and done by a single person, T.J.O.). However, closer inspection of frequency distributions of the data for each robot revealed that sampling across light conditions was not always uniform for all robots, resulting in additional interactions with Robot ID. To examine the consequences of this, we repeated our analyses on data collected with the robot used in most playbacks (Robot A: 47%, 154 of 331 playbacks). These results were qualitatively the same to analyses based on data from all robots. This analysis also helped clarify the interaction between display-type and robot ID in the extreme-receiver distance experiment (Table 2B). Responses to each robot were generally consistent to all display types, but those to Robot A performing the “alert, novel” display were particularly slow (which is also reflected in the main effect for display type in Table 2B and the slower responses for this “alert, novel” in bright light in Fig. 2; see also Fig. S2). This finding did not change the main conclusions of experiment 2.
Discussion
We provide evidence that animals facultatively include a special “alerting” component before the “message” component of their communication signals under environmental conditions in which receivers are less likely to detect those signals. In addition, the playback experiments indicate that alerting movements improve the ability of receivers to detect signals under adverse (low light) conditions when those recipients are located at distances typical for communication in A. gundlachi, but not when recipients are located at distances that exceed those at which communication would typically occur. The advantage conferred by an alert is therefore tightly linked to both the current conditions in the environment and to receiver distance. The attention-grabbing bob patterns that these lizards use as alerts would presumably be obvious to visually oriented predators as well (e.g., the Puerto Rican lizard-eating cuckoo (Coccyzus vieilloti) and feral mongoose (Herpestes javanicus) both prey on anoles (28, 29) and have been observed to attack displaying lizards and exaggerated pushups may also be energetically expensive to produce (30, 31). As was noted above, costs of alert production would encourage the use of alerts to situations and distances where they are necessary for conspecific signal detection, as we demonstrate here.
Interestingly, lizards responded to displays with alerts more quickly under low light than under bright light (Fig. 2). An additional experiment showed that this result is consistent with the hypothesis that lizards attend more closely to stationary models of conspecifics under poor light than under bright light. This could be because the position and behavior of conspecifics are easier to monitor under bright than poor light conditions. Lizards may also attend more to conspecific displays in low light because under natural conditions male Anolis lizards tend to display at the highest rates at dawn and dusk (32), when ambient light also tends to be low. Alternatively, the abrupt, high-speed movement of alerts may be especially suited at attracting attention in low light because this type of movement is better at stimulating the visual system of anoles in low light than in bright light. In addition, because display movements could also attract the attention of predators, lizards might limit conspicuous communication to situations in which predators are less likely to detect their displays.
There is growing evidence that animals regulate signal qualities to compensate for environmental noise [e.g., adjusting the dominant frequency of vocal signals (4, 22); or the speed (6) or duration (16) of visual displays], or omit conspicuous display elements when they are in the presence of predators (23, 24). However, changing the design of signals by adding or subtracting components to compensate for challenging environmental conditions has not previously been documented. Descriptions in the literature (10, 12, 14, 15, 17, 18) and the only two experimental investigations of alerts that we know of [one acoustic (11), the other visual (16; see also ref. 26)] imply that the evolution of alert components is potentially prevalent, yet the phenomenon remains largely unexamined. If putative alerts are verified in other systems, the strategy will represent an extraordinary example of functional convergence in animal communication crossing signal modalities (acoustic and visual) and taxonomic boundaries (mammals, frogs, birds, and lizards).
Methods
Alert Production by Free-living Lizards.
In 2006, a wild population of A. gundlachi was studied from April 4 to May 5 (corresponding to the start of the peak activity season) in the shade forest near the El Verde Field Station in the Caribbean National Forest on Puerto Rico. Focal lizards were video-recorded with a Panasonic GV-500 digital camcorder positioned on a tripod ≈4–5 m away from the focal lizard for an initial period of 10 min. If a lizard failed to display during this time, the trial was scrapped. In most instances lizards produced broadcast displays, and video-recording continued for a total of 25–30 min or until the lizard moved out of view. At the end of this recording period, habitat light was measured at the site of first display by using a LI-250A light meter fitted with a LI-190SA Quantum Sensor (LI-COR, Inc.). Light readings were taken with the sensor positioned horizontal, averaged over 15 s and then again across two readings corresponding to the approximate position of the left and right eye. Following light measurements, the distances to all of the conspecific males in the line of sight of the focal were measured using a tape measure and used to compute the average distance to visible neighbors for each focal animal. Females may also be potential recipients of broadcast displays, but because males typically establish larger territories that overlap the smaller home ranges of adult females (33) the distribution of males in the surrounding habitat can be expected to encompass the positions of most potential female receivers as well.
For the 38 adult males observed, the proportion of displays beginning with 4-legged pushups was scored from videotape and was multiplied by the average number of displays produced per minute to provide a weighted index factoring for overall display production. For example, an individual that produced 10 displays over 25 min is provided more weight than an individual that produced only one display during the same period (a multiplication factor of 0.4 versus 0.04, respectively). The rationale here was that animals observed to produce many broadcast displays provide a more accurate picture of when introductory pushups are used than individuals that performed few displays. As an alternative, we also examined the unweighted proportion of displays with alerts, transformed to meet the assumptions of normality by using a modified version of the Freeman and Tukey method presented by Zar (34), in a multiple regression analysis that included the number of displays produced by focal animals as a covariate. Results were comparable to those reported in Table 1 (overall model, r2 = 0.59; mean distance to neighbors, partial r = 0.36; visual noise, partial r = 0.50; habitat light, partial r = −0.67). We focus on analyses using the weight index as it provides a clearer picture of the factors influencing alert production.
Visual noise was estimated from the amount of movement occurring in the background of videos and categorized as either low (still to light) or high (moderate to windy). To provide a benchmark for these estimates, we referred back to a previous study (6) that used computational motion analysis software (35) to quantify precisely the speed of environmental motion occurring in video backgrounds of displaying lizards from this same population of A. gundlachi recorded previous to the present study in 2005. Clips from this previous study provided examples of low and high amounts of visual noise (below and above quartiles of population background speed estimates) and were referred to while making qualitative assessments of visual noise for the present study. This allowed video clips to be categorized far more quickly than formal motion analysis (i.e., a day of reviewing clips versus several weeks of intensive video processing). More important, we wanted to adopt the same method used for categorizing visual noise during robot playback experiments (see next section), where motion analysis software could not be used because of complications in calibrating estimates across trials.
Before statistical analysis, we confirmed that all continuous variables satisfied assumptions of normality. This lead to a log10(x + 1)-transformation for alert production and habitat light [Kolmogorov–Smirnov test: log10(alert production + 1), D38 = 0.11, P > 0.20; log10(light + 1), D38 = 0.15, P = 0.02, minus an outlying male highlighted in box-plots (see below) D37 = 0.12, P = 0.16; average distance to all neighbors, D27 = 0.10, P > 0.20]. These and all other statistical analyses were performed by using SPSS v. 11.5. Box-plots for both light and neighbor distance revealed outlying estimates for one focal lizard (see Fig. 1). The removal of this lizard from the dataset had no qualitative effects on the findings of our multiple regression analysis.
Alert Detection by Receivers.
In 2007, from June 6 to July 30 (midactivity season), we returned to the same wild population of A. gundlachi near the El Verde Field Station studied in 2005/2006 and conducted robot playbacks to test whether the addition of alerts at the start of broadcast displays enhanced orientation responses by receivers.
Robot construction and programming.
We built three robotic lizards, each consisting of a latex model taken from casts of a known territory holder removed from the population in 2006. Based on the snout-vent lengths of territorial males in the population recorded during the 2006 observational study (mean ± 1 SE = 64.3 ± 0.6 mm, range = 60–69, n = 21), three males of lengths 67, 68, and 69 mm, respectively, were used to make casts for the robots. Each model was attached to two step-motors (1.8-inch linear actuator, HIS 46000 series; Hayden Switch & Instrument, Inc.), one of which manipulated the up/down movement of the body, the other the extension/retraction of a colored fabric dewlap (color-matched to the dewlaps of live A. gundlachi by using a spectrophotometer); both were controlled separately by a compact integrated driver (MicroLynx-4, MX-CS 100–401; Intelligent Motion Systems, Inc.). The entire apparatus was powered by 32 rechargeable AA batteries hidden below the model along with the rest of the mechanics inside a waterproof plastic container (specifically, a Rubbermaid cookie jar). A quick release tripod mount was fitted to the side of the container to allow the robot to be positioned quickly on a tripod, nose down and side-on to the focal lizard with the container hidden behind a tree. Display treatments were rotated between the three robots.
To develop the display programs for the robots, we used our library of video footage collected in 2006 to quantify the display movements of free-living adult male A. gundlachi. Display clips were edited from digital tape by using iMovie HD 6.0.3 and exported as QuickTimes. Display-action-pattern graphs (DAP-graphs) depicting the change in head position over time were then created through frame-by-frame analysis by using NIH ImageJ 1.37v. These data were converted from pixels to mm following Ord et al. (6). Of the 38 males video-recorded, DAP-graphs of 135 displays for 31 males (median displays per individual: 4; range: 1–9) were obtained in which no camcorder movement occurred and the lizard was in full view. For the species-typical portion of the robot display (the “message” component), we selected a representative display from an adult male and measured the temporal and amplitude parameters from the DAP-graph. This male was subsequently used for making model casts but was otherwise not included in playback experiments. We developed this part of the robot program from a representative display rather than using a “population median” display (see below) because the structure of this portion of the display is particularly complex (Fig. S2) and potentially an important source of cues about the identity and motivation of the signaler. We do not know yet how manipulating this “message” portion of the display would affect the behavior of receivers. To circumvent this issue, we chose a representative display to ensure that the natural integrity of these cues was retained.
To create the alert introductions, we measured the amplitude and the duration of pushups from DAP-graphs (Tables S2 and S3). These data were summarized as median values and used to program the opening sequence of the “alert, typical” display (Fig. S2). The number of pushups (“alert, typical”) or rapid dewlaps (“alert, novel”) performed by the robot was based on the population median of 6 rapid introductory movements, corresponding to 2 or 3 pushups (depending on the start position of the animal; whether pushups began from a raised or resting posture close to the substrate). All robots were programmed to perform displays every 88.6 seconds, corresponding to the median interdisplay interval for this population (the period from the end of one display to the beginning of the next). Interdisplay intervals (range: 12.8–257.6 s) were measured to the nearest video frame (33 ms) from video records of 29 males that performed two or more displays.
Playback experiments.
Our goal for the first playback experiment (“typical-receiver distance”) was to approximate the average distance of most receivers (5.5 ± 2.56 m, mean ± 1 SD, n = 27). However, distances from the robot to the focal lizards were constrained by the location of trees suitable for robot placement, namely trees large enough to hide the tripod (larger than ≈20 cm in diameter), within the line of sight of the focal animal (not obscured by foliage or other visual obstructions), and lacking conspecific males or females perched on them at the time of the experiment. The average distance of the robot for the “typical receiver distance” presentations was 4.5 ± 1.11 m (n = 159). This distance tended to be a bit closer than the average neighbor distance estimated for free-living lizards in our initial study [unequal-variance t test: t27.7 = 1.912, P = 0.07; 95% confidence interval (CI) for effect size d = −0.01–0.81; both neighbor and robot distance data satisfied assumptions of normality for a t test (Kolmogorov–Smirnov test: neighbor distance, D27 = 0.10, P > 0.20; robot distance, D159 = 0.06, P > 0.20)], but the 95% CIs of mean robot and neighbor distances overlapped (neighbors: 4.5–6.5 m, n = 27 focal lizards; robots: 4.4–4.7 m, n = 172 presentations). Furthermore, the minimum and maximum distance of the robot (2.4–8.0 m) was within the 10th to 90th percentile bounds of average neighbor distances (2.2–8.0 m; see Fig. S3). Our presentation distances were therefore a reasonable approximation of typical-receiver distances for A. gundlachi.
Our goal for the second playback experiment (“extreme-receiver distance”) was to position the robot at a distance at which focal lizards would be able to see, but unlikely to interact with, another male. We based this distance on the maximum distance recorded in our initial study for a male conspecific within the line of sight of a focal lizard, 12.6 m. For this experiment, the average distance of robots (mean ± 1 SD = 12.8 ± 1.82 m, n = 172) was comparable to the maximum distance estimated for a male neighbor [one sample t test: t171 = 1.65, P = 0.10, 95% CI of effect size d = 0.03–0.27; robot distance met the assumption of normality for a t test (Kolmogorov–Smirnov test: D172 = 0.05, P > 0.20)]. The CIs for mean robot distance also encompassed the 12.6 m estimate for maximum neighbor distance (12.5–13.1 m, n = 184 presentations). Fig. S3 shows the relationship between alert use and neighbor distance from the observational study conducted in the previous year (see previous section) and how these data were used to guide the presentation distances for the robots in the playback experiments. This figure also illustrates the bimodal, nonoverlapping distribution of robot placements used for the typical-receiver and extreme-receiver distance presentations.
From video records of playbacks, “orientation time” was measured as the length of time (estimated to the nearest video frame, 33 ms) from the onset of the first display movement by the robot to the first head movement of the focal lizard toward the robot. The measurement of responses was blind to the experimental treatment (presence or absence of alerting signals). During the initial 7-min acclimatization period when the robot was stationary, most of the focal animals oriented toward the robot at some point. Our measure of orientation response therefore does not indicate the first time the focal lizards oriented toward the robot, but rather the first time that the focal lizards oriented to the robot after it began to display.
Protocols for measuring visual noise and habitat light were the same as those outlined in the previous section. Briefly, visual noise was categorized from video during the first 2 min from the onset of the first display movement by the robot. Habitat light was measured directly at the site the focal lizard was first observed and generally taken within 10 min of the focal lizard”s first orientation response (i.e., at the end of the playback presentation).
Before statistical analysis, the time focal lizards took to orient toward the robot and habitat light were log10(x + 1)-transformed to meet the assumptions of normality [Kolmogorov–Smirnov test: log10(orientation time + 1), D327 = 0.04, P > 0.20; log10(light + 1), D327 = 0.04, P > 0.20]. Box-plots for each experiment were inspected for outlying data points. Four outliers which correspond to focal lizards that viewed the robot in unusually bright conditions—one lizard participating in the first experiment (typical-receiver distance) and three in the second experiment (extreme-receiver distance)—were highlighted in box-plots, but their removal yielded the same qualitative results as in the initial analyses. Figs. S4 and S5 illustrate orientation time as a function of the viewing conditions experienced by each lizard during playbacks at typical-receiver and extreme-receiver distances, respectively.
Rapid receiver responses to alerts in poor light.
Our playback results indicated that at typical-receiver distances, lizards responded to alert displays more quickly under low light than in bright light (Fig. 2 and Fig. S4). We used data collected in another experiment conducted on the same population of A. gundlachi in 2007 to examine this relationship further (see Table S1 and Fig. S1). We compared the number of head movements directed toward still robots to the number of head movements directed at a control object, which consisted of a small bunch of plastic leaves, trimmed to cover about the same area as the robot. As with other playback experiments, the still robot or the control object was placed on a tripod behind a tree, level with the focal lizard and at a distance typical of most neighbors (still robot, mean ± 1 SD: 4.1 ± 0.9 m, n = 94; control: 4.4 ± 1.3, n = 56). Once the stimulus had been positioned, a cover concealing it was removed and we video-recorded the focal lizard for the following 7 min. All focal animals returned to normal activity (e.g., foraging, displaying, moving about their territory) within 2-min, so we focused on the focal lizard”s behavior from 2 to 7 min after the cover was removed from the stimulus object. At the end of trials, habitat light was measured at the site where the focal lizard was first observed. The total number of head-turns toward stimuli during the last 5 min was scored blind from video (all but two focal lizards oriented at least once toward the stimuli). Data were checked for normality and transformed accordingly [Kolmogorov–Smirnov test: √(total number of head-turns toward stimulus), D150 = 0.06, P > 0.20; log10(light + 1), D150 = 0.06, P > 0.20].
Supplementary Material
Acknowledgments.
We thank Zac Costa, Alexis Harrison, Daniel Hooper, Shannon Seil, and Clare Steinberg for assistance in the field (Zac Costa also helped create the extensive DAP-graph library on which robot programs were based), Leo Fleishman for his help with the robot dewlap and advice on appropriate measures of habitat light, and Alonzo Ramirez and the University of Puerto Rico for providing access to facilities at the El Verde Field Station. We are also indebted to Tonia Hsieh, Jonathan Losos, Richard Peters, Haven Wiley, and two anonymous reviewers for comments on a previous version of this manuscript. This work was supported by grants from the National Geographic Society and the US National Science Foundation Grant IOB-0517041/0516998, and was covered by the Animal Use and Care Protocol 05–11652 initially approved on March 24, 2005 and reviewed again on March 22, 2007 by the Institutional Animal Care and Use Committee of the University of California, Davis.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807657105/DCSupplemental.
References
- 1.Alexander RD. Evolutionary change in cricket acoustical communication. Evolution. 1962;16:443–467. [Google Scholar]
- 2.Marler P. Animal communication signals. Nature. 1967;157:769–774. doi: 10.1126/science.157.3790.769. [DOI] [PubMed] [Google Scholar]
- 3.Morton ES. Ecological sources of selection on avian sounds. Am Nat. 1975;109:17–34. [Google Scholar]
- 4.Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. [DOI] [PubMed] [Google Scholar]
- 5.Feng AS, et al. Ultrasonic communication in frogs. Nature. 2006;440:333–336. doi: 10.1038/nature04416. [DOI] [PubMed] [Google Scholar]
- 6.Ord TJ, Peters RA, Clucas B, Stamps JA. Lizards speed up visual displays in noisy motion habitats. Proc R Soc Lond B. 2007;274:1057–1062. doi: 10.1098/rspb.2006.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal M, Fleishman LJ. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc R Soc Lond B. 2002;269:351–359. doi: 10.1098/rspb.2001.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong BBM, Candolin U, Lindstrom K. Environmental deterioration compromises socially enforced signals of male quality in three-spined sticklebacks. Am Nat. 2007;170:184–189. doi: 10.1086/519398. [DOI] [PubMed] [Google Scholar]
- 9.Gray SM, et al. Environment-contingent sexual selection in a colour polymorphic fish. Proc R Soc Lond B. 2008;275:1785–1791. doi: 10.1098/rspb.2008.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiley RH, Richards DG. In: Acoustic Communication in Birds. Kroodsma DE, Miller EH, Ouellet H, editors. Vol 1. New York: Academic; 1982. pp. 131–181. [Google Scholar]
- 11.Richards DG. Alerting and message components in songs of rufous-sided towhees. Behaviour. 1981;76:223–249. [Google Scholar]
- 12.Brenowitz EA. Long-range communication of species identity by song in the red-winged blackbird. Behav Ecol Sociobiol. 1982;10:29–38. [Google Scholar]
- 13.Bloomfield LL, Farrell TM, Sturdy CB. All “chick-a-dee” calls are not created equally Part II. Mechanisms for discrimination by sympatric and allopatric chickadees. Behav Proc. 2008;77:87–99. doi: 10.1016/j.beproc.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Gerhardt HC. Significance of two frequency bands in long distance vocal communication in the green treefrog. Nature. 1976;261:692–694. [Google Scholar]
- 15.Mitchell BR, Makagon MM, Jaeger MM, Barrett RH. Information content of coyote barks and howls. Bioacoustics. 2006;15:289–314. [Google Scholar]
- 16.Peters RA, Hemmi JM, Zeil J. Signaling against the wind: Modifying motion-signal structure in response to increased noise. Curr Biol. 2007;17:1231–1234. doi: 10.1016/j.cub.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 17.Fleishman LJ. The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am Nat. 1992;139:S36–S61. [Google Scholar]
- 18.Stamps JA, Barlow GW. Variation and stereotypy in the displays of Anolis aeneus (Sauria: Iguanidae) Behaviour. 1973;47:67–94. [Google Scholar]
- 19.Fleishman LJ. Motion detection in the presence and absence of background motion in an Anolis lizard. J Comp Physiol A. 1986;159:711–720. doi: 10.1007/BF00612043. [DOI] [PubMed] [Google Scholar]
- 20.Persons MH, Fleishman LJ, Frye MA, Stimphil ME. Sensory response patterns and the evolution of visual signal design in anoline lizards. J Comp Physiol A. 1999;184:585–607. [Google Scholar]
- 21.Foote AD, Osborne RW, Hoelzel AR. Whale-call response to masking boat noise. Nature. 2004;428:910. doi: 10.1038/428910a. [DOI] [PubMed] [Google Scholar]
- 22.Gillam EH, McCracken GF. Variability in the echolocation of Tadarida brasiliensis: Effects of geography and local acoustic environment. Anim Behav. 2007;74:277–286. [Google Scholar]
- 23.Rand AS, Ryan MJ. The adaptive significance of a complex vocal repertoire in a neotropical frog. Z Tierpsychol. 1981;57:209–214. [Google Scholar]
- 24.Ryan MJ, Tuttle MD, Rand AS. Bat predation and sexual advertisement in a neotropical anuran. Am Nat. 1982;119:136–139. [Google Scholar]
- 25.Fleishman LJ, Marshall CJ, Hertz PE. Comparative study of temporal response properties of the visual system of three species of anoline lizards. Copeia. 1995;1995:422–431. [Google Scholar]
- 26.Peters RA. Environmental motion delays the detection of movement-based signals. Biol Lett. 2008;4:2–5. doi: 10.1098/rsbl.2007.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins EP, Ord TJ, Davenport SW. Combining motions into complex displays: Playbacks with a robotic lizard. Behav Ecol Sociobiol. 2005;58:351–360. [Google Scholar]
- 28.Reagan DP, Camilo GR, Waide RB. In: The Food Web of a Tropical Rain Forest. Reagan DP, Waide RB, editors. Chicago: University of Chicago Press; 1996. pp. 461–510. [Google Scholar]
- 29.Vilella FJ. Biology of the mongoose (Herpestes javanicus) in a rain forest of Puerto Rico. Biotropica. 1998;30:120–125. [Google Scholar]
- 30.Brandt JM. Lizard threat display handicaps endurance. Proc R Soc Lond B. 2003;270:1061–1068. doi: 10.1098/rspb.2003.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry G, Levering K, Girard I, Garland T., Jr Locomotor performance and social dominance in male Anolis cristatellus. Anim Behav. 2004;67:37–47. [Google Scholar]
- 32.Ord TJ. Dawn and dusk ‘chorus’ in visually communicating Jamaican anole lizards. Am Nat. 2008;172:585–592. doi: 10.1086/590960. [DOI] [PubMed] [Google Scholar]
- 33.Stamps JA. In: Lizard Ecology: Studies of a Model Organism. Huey RB, Pianka ER, Schoener TW, editors. Cambridge, Massachusetts: Harvard Univ Press; 1983. pp. 169–204. [Google Scholar]
- 34.Zar JH. Biostatistical Analysis. New Jersey: Prentice Hall; 1999. [Google Scholar]
- 35.Peters RA, Clifford CWG, Evans CS. Measuring the structure of dynamic visual signals. Anim Behav. 2002;64:131–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.