Abstract
Schistosoma mansoni worm eggs stimulate a T-cell-mediated granulomatous response in which macrophages play important inflammatory and regulatory roles. Although much has been learned about the functions of the schistosome granuloma macrophages, their origin and replicative ability are unknown. In the present sequential study, macrophage progenitor cells in the bone marrow (GM-CFC) and liver granulomas (M-CFC) were enumerated, and macrophage colony-stimulating factor (CSF-1) in the circulation and culture fluid of explanted granulomas of infected mice was assayed. During the acute phase of the infection, when the granulomatous response was vigorous (weeks 8 to 12) GM-CFC numbers were high in the bone marrow and M-CFC numbers were low within granulomas. Circulating CSF-1 levels were elevated, but the vigorous granuloma-secreted CSF-1 level was low. During the chronic phase of the infection, the number of GM-CFC within the bone marrow and levels of circulating CSF-1 returned to normal. Conversely, a sharp increase in the number of M-CFC occurred within the small immunomodulated granulomas that also secreted high levels of CSF-1. The frequency of M-CFC that proliferated under exogenously added CSF-1 within the immunomodulated granulomas was significantly higher than that of cells in the vigorous granulomas. Adherent macrophage-rich cells obtained after dispersal of the granulomas appeared to be one source of CSF-1 production. These data indicate that during the infection the macrophage supply to and replicative ability within the granulomas is influenced by systemically and/or locally produced CSF-1.
Full text
PDF
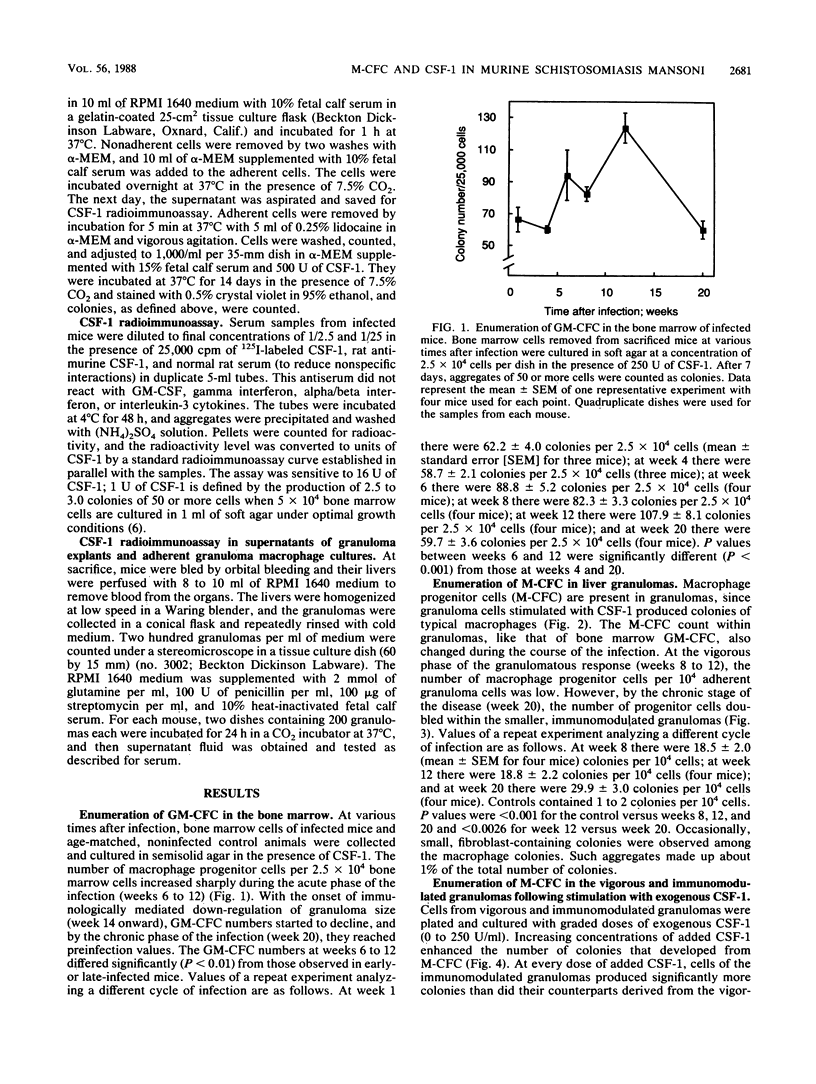
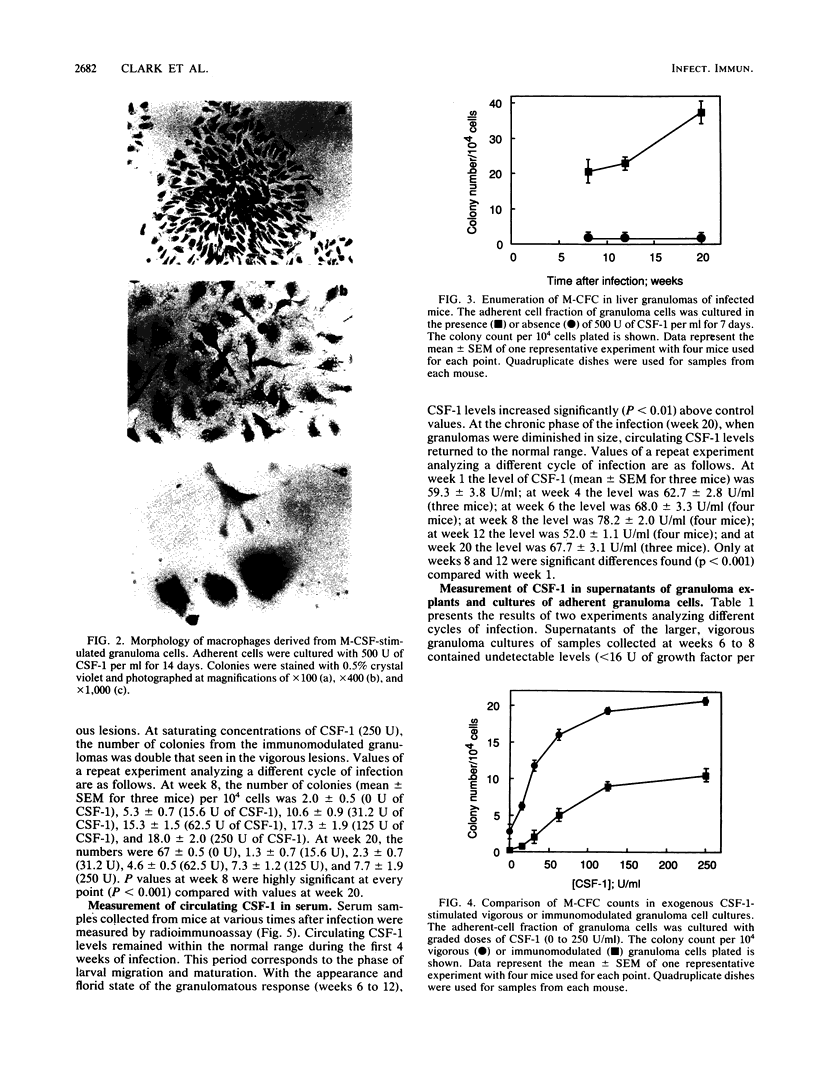
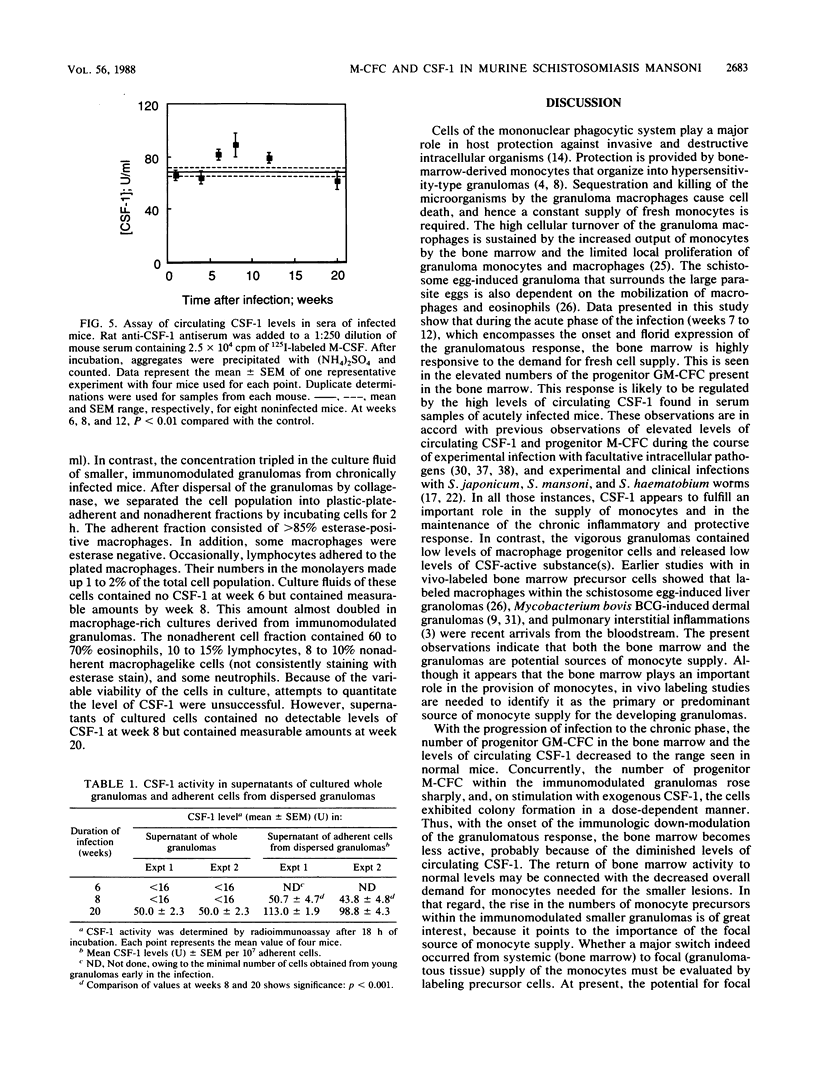


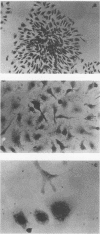
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsden A. F., Boros D. L. Fc-receptor-bearing macrophages isolated from hypersensitivity and foreign-body granulomas. Delineation of macrophage dynamics, fc receptor density/avidity and specificity. Am J Pathol. 1979 Aug;96(2):457–476. [PMC free article] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., Mattie H., van Furth R. A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet. 1983 May;16(3):211–219. [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., Van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am Rev Respir Dis. 1983 Aug;128(2):276–281. doi: 10.1164/arrd.1983.128.2.276. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L. Immunoregulation of granuloma formation in murine schistosomiasis mansoni. Ann N Y Acad Sci. 1986;465:313–323. doi: 10.1111/j.1749-6632.1986.tb18507.x. [DOI] [PubMed] [Google Scholar]
- Chen B. D., Clark C. R. Interleukin 3 (IL 3) regulates the in vitro proliferation of both blood monocytes and peritoneal exudate macrophages: synergism between a macrophage lineage-specific colony-stimulating factor (CSF-1) and IL 3. J Immunol. 1986 Jul 15;137(2):563–570. [PubMed] [Google Scholar]
- Chensue S. W., Kunkel S. L., Higashi G. I., Ward P. A., Boros D. L. Production of superoxide anion, prostaglandins, and hydroxyeicosatetraenoic acids by macrophages from hypersensitivity-type (Schistosoma mansoni egg) and foreign body-type granulomas. Infect Immun. 1983 Dec;42(3):1116–1125. doi: 10.1128/iai.42.3.1116-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Elliott D. E., Boros D. L. Schistosome egg antigen(s) presentation and regulatory activity by macrophages isolated from vigorous or immunomodulated liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1984 Mar;132(3):1506–1510. [PubMed] [Google Scholar]
- Elliott D. E., Righthand V. F., Boros D. L. Characterization of regulatory (interferon-alpha/beta) and accessory (LAF/IL 1) monokine activities from liver granuloma macrophages of Schistosoma mansoni-infected mice. J Immunol. 1987 Apr 15;138(8):2653–2662. [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. The pulmonary macrophage in acute leukemia. N Engl J Med. 1974 Apr 18;290(16):875–878. doi: 10.1056/NEJM197404182901603. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Kuhn C., 3rd, Chen D. M. Effects of hydrocortisone acetate on pulmonary alveolar macrophage colony-forming cells. Am Rev Respir Dis. 1982 Jun;125(6):712–715. doi: 10.1164/arrd.1982.125.6.712. [DOI] [PubMed] [Google Scholar]
- Loveless S. E., Wellhausen S. R., Boros D. L., Heppner G. H. Tumoricidal macrophages isolated from liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1982 Jan;128(1):284–288. [PubMed] [Google Scholar]
- Mahmoud L. A., Robinson W. A., Entringer M. A. Urinary granulocyte colony-stimulating factor in bilharziasis. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):518–521. doi: 10.4269/ajtmh.1982.31.518. [DOI] [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Regulation of macrophage production. Adv Exp Med Biol. 1982;155:33–48. doi: 10.1007/978-1-4684-4394-3_3. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Owhashi M., Nawa Y. Granulocyte-macrophage colony-stimulating factor in the sera of Schistosoma japonicum-infected mice. Infect Immun. 1985 Sep;49(3):533–537. doi: 10.1128/iai.49.3.533-537.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T. The significance of local resident pulmonary alveolar macrophage proliferation to population renewal. J Leukoc Biol. 1986 Jan;39(1):77–87. doi: 10.1002/jlb.39.1.77. [DOI] [PubMed] [Google Scholar]
- Schook L. B., Wellhausen S. R., Boros D. L., Niederhuber J. E. Accessory cell function of liver granuloma macrophages of Schistosoma mansoni-infected mice. Infect Immun. 1983 Dec;42(3):882–886. doi: 10.1128/iai.42.3.882-886.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadecker M. J., Wright J. A. Distribution and kinetics of mononuclear phagocytes in granulomas elicited by eggs of Schistosoma mansoni. Am J Pathol. 1984 Aug;116(2):245–252. [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Cifone M., Heard P. M., Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976 Mar 1;143(3):631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarling J. D., Coggle J. E. The absence of effect on pulmonary alveolar macrophage numbers during prolonged periods of monocytopenia. J Reticuloendothel Soc. 1982 Mar;31(3):221–224. [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T., Dannenberg A. M., Ando M., Abbey H., Corrin A. R. Mononuclear cell turnover in chronic inflammation: studies on tritiated thymidine-labeled cells in blood, tuberculin traps, and dermal BCG lesions of rabbits. Am J Pathol. 1976 May;83(2):255–268. [PMC free article] [PubMed] [Google Scholar]
- Van Furth R., Diesselhoff-den Dulk M. C., Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973 Dec 1;138(6):1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. The secret of the immunopathogenesis of schistosomiasis: in vivo models. Immunol Rev. 1982;61:189–213. doi: 10.1111/j.1600-065x.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Wellhausen S. R., Boros D. L. Comparison of Fc, C3 receptors and Ia antigens on the inflammatory macrophage isolated from vigorous or immunomodulated liver granulomas of schistosome-infected mice. J Reticuloendothel Soc. 1981 Sep;30(3):191–203. [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. C., Waheed A., Shadduck R. K. Effect of Listeria monocytogenes infection on serum levels of colony-stimulating factor and number of progenitor cells in immune and nonimmune mice. Infect Immun. 1985 Aug;49(2):325–328. doi: 10.1128/iai.49.2.325-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Magee D. M., Barczynski L. K. Analysis of colony-stimulating factors and macrophage progenitor cells in mice immunized against Listeria monocytogenes by adoptive transfer. Infect Immun. 1987 Aug;55(8):1843–1847. doi: 10.1128/iai.55.8.1843-1847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-den Dulk M. M. Dual origin of mouse spleen macrophages. J Exp Med. 1984 Nov 1;160(5):1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]