Abstract
Ethanol has been known to cause injury to the liver and other tissues; however the molecular factors responsible for alcohol induced liver injury has not been fully understood. Recent studies indicate that reactive oxygen species (ROS) may play an important role in alcohol induced liver injury. Peroxisome proliferator activated receptor-γ (PPAR-γ)-coactivator 1α (PGC-1α) has been shown to be involved in defenses against ROS by inducing many ROS-detoxifying enzymes. However, the role of PGC-1α in alcohol induced liver injury has not been elucidated. Therefore, in this study, we examined the effect of alcohol on gene and protein expression of PGC-1α in H4-IIE cells (in vitro) and hepatic tissues (in vivo) by real-time PCR and Western blot, respectively. Our results show that exposure to 500 mM ethanol in H4-IIE cells for 24 h significantly decreased both gene and protein expression of PGC-1α. PGC-1α gene expression was significantly decreased in cells exposed to 100 ng/ml LPS or 1% hypoxia for 24 h. In addition, PGC-1α gene and protein expressions were slightly lower in hepatic tissues of rats exposed to ethanol for 15 h, at the level equivalent to the 500 mM used in culture cells, in comparison to sham rats. In contrast, serum LDH and AST levels in ethanol exposed rats were 1.9 fold and 2.8 fold higher than that of sham rats, respectively, which suggest significant organ injury in these rats following ethanol exposure. Likewise, catalase, an enzyme that hydrolyzes peroxide to water, is significantly increased in ethanol exposed H4-IIE cells which further confirms ROS generation due to ethanol exposure. Thus, our results show that oxidative stress conditions such as acute alcohol consumption, LPS or hypoxia suppresses PGC-1α expression in the liver, thereby presumably downregulates pertinent ROS-scavenging enzymes and enhances liver injury.
Keywords: Alcohol, PGC-1α, liver, H4-IIE cells, LPS, hypoxia
Introduction
Excessive alcohol consumption is the most prevalent cause of liver injury related morbidity and mortality in the United States. It is the third leading cause of controlled death in the United States with 12,000 deaths each year contributing to alcoholic liver disease [1]. The pathogenesis of chronic and acute alcohol consumption is complex and multifactorial with diverse consequences in different tissues and cell types. Alcohol induced liver injury is marked by pathological changes in the liver ranging from steatosis, steatohepatitis to cirrhosis and sometimes hepatocellular carcinoma. Chronic alcohol consumption leads to elevated endotoxin levels in the blood and the liver due to an increase in gut permeability [2]. Endotoxin, recognized by Kupffer cells in the liver, causes the release of pro-inflammatory cytokines and chemokines which produce systemic inflammatory responses [2-4]. In this regard, elevated serum concentrations of TNF-α, IL-6 and IL-8 have been reported in alcoholic patients [5]. In addition to cytokines, Kupffer cells release reactive oxygen species (ROS) which negatively affects hepatocyte function. Hepatocytes undergoing oxidative stress due to ROS are sensitized to TNF-α induced apoptosis and necrosis [6,7]. Thus, it is widely accepted that ROS not only causes direct hepatocyte injury but also contributes to increased inflammatory responses which in turn, causes further liver injury. A significant target of alcohol induced ROS is mitochondrial damage which leads to mitochondrial dysfunction and causes lipid peroxidation and protein modification [8].
The molecular mechanism of alcohol induced liver injury has not been completely understood. A large body of evidence shows the involvement of transcription factors in alcoholic liver injury [9-13]. Similarly, activation of inflammatory mediators, NRcB [14,15] and AP-1 [16] have also been shown in alcohol induced liver injury. It has been shown that peroxisome proliferator activated receptor-γ (PPAR-γ), another transcription factor known to inhibit inflammatory responses, is also regulated by the chronic alcohol exposure in Kupffer cells and hepatocytes [17,18]. Treatment with, PPAR-γ agonists prevented the development of chronic alcohol induced steatosis and inflammation [19]. Recently, it has been shown that PGC-1α, a well established coactivator of PPAR-γ, is essential for the induction of many ROS-detoxifying enzymes including GP×1 and superoxide dismutase 2 (SOD2) and is considered as a broad and powerful regulator of ROS metabolism [20-22]. PGC-1α, a 92-kDa transcription factor originally identified as a co-activator of PPAR-γ, is now recognized as the master regulator of many biological processes which include mitochondrial biogenesis, thermogenesis, gluconeogenesis and energy production [reviewed in [23-25]]. PGC-1α was originally described as a cold-inducible co-activator controlling adaptive thermogenesis in brown adipose tissue and skeletal muscle by stimulating mitochondrial biogenesis and oxidative metabolism [26]. Hepatic PGC-1α expression induced by fasting has shown to increase gluconeogenesis. In contrast, induction of PGC-1α due to exercise is associated with mitochondrial biogenesis and respiration in skeletal muscle and cardiac muscles. These functional diversities of PGC-1α in different tissues are accomplished by the activation of nuclear receptors like PPARs, thyroid hormone receptors, estrogen-related receptors, glucocorticoid receptor, hepatocyte nuclear factor 4α, nuclear respiratory factors and FOX01. These diverse functions appear to be specific for different tissues.
Excessive generation of ROS plays an important role in alcohol induced cellular damage [7]. A number of in vitro studies show that alcohol increases ROS generation [27-29]. However, the role of PGC-1α in alcohol induced liver injury has not been elucidated. Therefore in the present study, we evaluated the effect of alcohol on the hepatic PGC-1α gene and protein expression both in vitro and in vivo. The effect of other oxidative stress conditions such as hypoxia and LPS treatment on PGC-1α expression was also evaluated in H4-IIEcells.
Materials and Methods
Cell culture
H4-IIE cells from ATCC (Cat: CRL-1548, Manassas, VA), originated from Rattus norvegicus hepatoma, were plated in 12-well multiple plates at a density of 0.3×106 cells/ml of Eagle's Minimum Essential Medium containing non-inactivated 10% fetal bovine serum (FBS). Cells were incubated in a 5% C02 incubator at 37°C overnight before treatments.
Ethanol and LPS treatment
Overnight cultures of H4-IIE were washed with 1× PBS. New media containing 0, 100, 500 mM ethanol (E7023, sigma-Aldrich) or 100 ng/ml LPS ( Escherichia coli 0111:B4; Sigma-Aldrich) were added to the cells and incubated at 37°C for 24 h. Afterwards, RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) and used for real time PCR analysis. In duplicate experiments, cells treated with different concentrations of ethanol were washed once with 1× PBS and lysed in 10 mM Tris-HCI, pH 7.5, 100 mM NaCI, 50 mM EDTA, 50 mM EGTA, 1% Triton-X-100 with 2 mM Na orthovanadate, 0.2 mM Phenylmethylsufonylflouride, 2 μg/ml leupeptin and 2 μg/ml aprotinin. After centrifugation at 16,000 g for 10 min, the supernatant was collected and the protein concentration was determined by using Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA).
Exposure to hypoxia
Overnight cultures of H4-IIE were washed with 1× PBS and new media were added to cells. The hypoxic environment was created by placing the plate in a sealed Modular Incubator Chamber (Billups-Rothenberg Inc. Del Mar, CA) and purging the chamber with 1% oxygen, 5% carbon dioxide and 94% nitrogen (WELCO-CGI Gas Technology Inc., Newark, NJ) at a rate of 20 liters/min for 5 min. Subsequently, the chamber was disconnected from the gas source, sealed by closing plastic clamp and placed into a 37°C incubator for 24 h. Following the hypoxia exposure, RNA was extracted using TRIzol reagent and used for real-time PCR analysis.
Animals and ethanol treatment
Male adult Sprague-Dawley rats (250-320g) purchased from Charles River Laboratories (Wilmington, MA) were used in this study. All rats were housed in a temperature controlled room on a 12-hr light/dark cycle and fed a standard Purina rat chow diet for at least one week before experiment. The experiments described here were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Animal Care and Use Committee of The Feinstein Institute for Medical Research. Rats were anesthetized with 0.5-1.5% isoflurane inhalation and a bolus intravenous injection of ethanol at 1.75g / kg body weight (BW), followed by an intravenous infusion of 300 mg/kg/h of ethanol for 15 h which was administered using a pump as previously described [30]. The continuous infusion of ethanol via the alzet mini-pump for 15 h was performed without anesthesia using a specialized animal restraint which allowed free movement of the rats within the cage during the entire time period. Sham animals received similar treatment with the exception of the ethanol infusion. Even though the plasma alcohol concentration was not measured in our studies, it has been reported that plasma concentration in rats infused with intragastric administration of ethanol dose as described above averaged as 135 ± 12 mg/dL [31]. Immediately after the completion of infusion, animals were sacrificed; blood and liver tissue samples were collected and stored at -70° for further analysis.
Gene expression by real-time PCR
Total RNA (4 μg) extracted from hepatic tissues or H4-IIE cells were reverse-transcribed as previously described [32]. Gene expression was determined by real-time PCR technique. The primer pair, designed specific for rat PGC-1α mRNA sequences (GenBank accessions#: NM_031347) was the following: forward primer: 5'-ATG AGA AGC GGG AGT CTG AA-3'; reverse primer: 5'-ACG GTG CAT TAA TCA ATT TC-3'. The primer pair designed for rat catalase (GenBank accession#: NM_012520) was forward primer: 5'-CCA GCG ACC AGA TGA AGCA 3'; reverse primer: 5' TGG TCA GGA CAT CGG GTTTC 3'. Primer pair specific for GAPDH (GenBank accession#: M17701) used as internal gene reference, was the following: forward primer: 5'-ATG ACT CTA CCC ACG GCA AG-3' and reverse primer: 5'-CTG GAA GAT GGT GAT GGG TT-3'. Real-time PCR was performed using 7300 Real-Time PCR system (Applied Biosystems) with SYBR Green as detection dye. The reaction was carried out in a 25 Ml final reaction volume containing 0.08 umol concentration of each forward and reverse primer, 2 μl cDNA, 9.2 μl H20 and 12 μl SYBR Green PCR Mast Mix (Applied Biosystems). The thermal profile for the real-time PCR was 50°C for 2 min, 95°C for 10 min and followed by 40 cycles of 95°C for 15 seconds and 60 °C for 1 min. The gene expression was expressed as fold change from the GAPDH level which is calculated as 2-ΔΔCt. In addition, melting curve analysis was performed to make sure the specificity of PCR product in this experiment.
PGC-1α protein expression
Total proteins (50 μg) from hepatic tissues or 10 μg from H4-IIE cells were loaded on 4-12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and electrophoretically fractionated in MES-SDS running buffer (Invitrogen). The protein on the gel was then transferred to a 0.45-um nitrocellulose membrane, and blocked with 5% nonfat dry milk in 10 mM Tris-HCI with 0.1% Tween 20, pH 7.5 (TBST). The membrane was incubated with 1:1000 dilution of rabbit anti-PGC-1α polyclonal antibody (H-300, sc-13067, Santa Cruz Biotechnology, CA) overnight at 4°C followed by incubation in 1:10,000 HRP-linked anti-rabbit IgG for 1 h at room temperature. Mouse anti-β-actin monoclonal antibody (1:20,000; Sigma, Saint Louis, MO) was used as the loading control in this experiment. To reveal the reaction bands, the membrane was reacted with ECL Western blot detection system (Amersham, Piscataway, NJ) and exposed on X-ray film. Bio-Rad GS-800 Calibrated Densitometer analysis system (Bio-Rad, Hercules, CA) was used to quantitate the Western blots. This system can select the contour of the band, subtract the background and calculate the density.
Lactate dehydrogenase (LDH) release assay
Serum LDH concentration was measured using the colorimetric assay kit from Pointe Scientific, Inc (Lincoln Park, Ml) according to the manufacturer's instructions.
Aspartate aminotransferase (AST) analysis
Serum AST concentration was measured using the colorimetric assay kit from Pointe Scientific, Inc. (Lincoln Park, Ml) according to the manufacturer's instructions.
Statistical analysis
All data were expressed as mean ± SEM. The statistical analysis methods are one-way ANOVA with Student-Newman-Keuls test. Student's t-test was also used for two group data analysis. Differences in values were considered significant if P < 0.05.
Results
PGC-1α expression was inhibited by ethanol treatment in H4-IIE cells
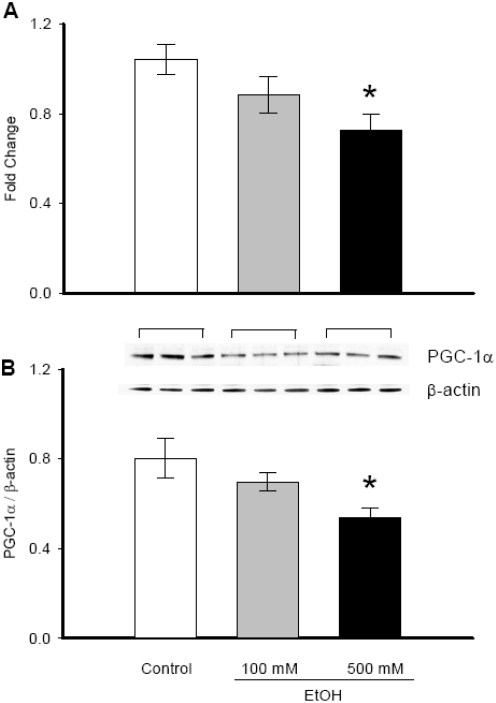
PGC-1α gene expression was significantly decreased when H4-IIE cells were treated with 500 mM ethanol for 24 h (1.041±0.07 vs. 0.725±0.07, P=0.003, Figure 1A). At 100 mM ethanol, PGC-1α gene expression was slightly lower than the untreated sample, however no statistical significance was evident. At 4 and 20 mM ethanol, PGC-1α gene expression was similar to untreated samples (data not shown). In parallel to the mRNA expression, at 500 mM ethanol, PGC-1α protein levels were decreased by 32.7% (P=0.015, Figure IB). At 100 mM ethanol, PGC-1α protein : expression was decreased by 13% but no statistical significance was observed. PGC-1α protein expression was also unchanged at 4 and 20 mM ethanol from the control (data not shown). In addition, MTS cell proliferation assay showed no toxic effect up to 500 mM ethanol on cells. However, at 1000 mM and above, ethanol treatment became toxic to the cells (data not shown).
Figure 1.
Alterations in PGC-1α gene and protein expression in ethanol treated H4-IIE cells. (A) RNA was extracted from H4-IIE cells treated with or without 100 mM or 500 mM ethanol treatment for 24 h. PGC-1α mRNA expression was measured by real-time PCR and presented as fold change over GAPDH levels. (B) Cells treated as above were lysed and subjected to Western blotting using anti-PGC-1α antibody. β-actin antibody was used as the internal control. Results are shown as a representative autoradiogram and the ratio between PGC-1α and β-actin expression (PGC-1α/β-actin). Data are presented as mean ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls method: * p<0.05 versus Control group.
Effects of LPS treatment or hypoxia exposure on PGC-1α gene expression in H4-IIE cells
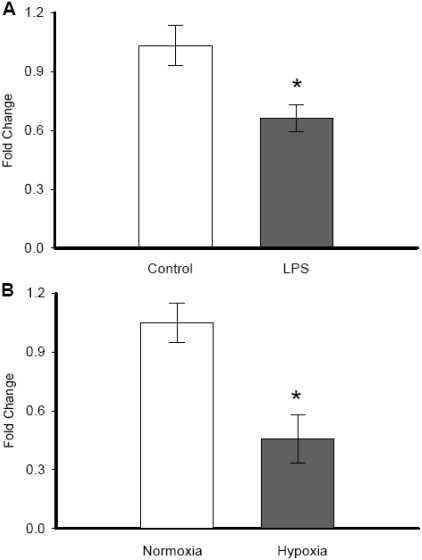
We also investigated the PGC-1α gene expression pattern associated with endotoxemia by administering LPS to the cells or with hypoxia condition by exposing cells to very low (1%) oxygen condition (Figure 2). PGC-1α gene expression was significantly decreased by 36.0% when cells were treated with 100 ng/ml bacterial LPS (1.033±0.10 vs. 0.661±0.07, P=0.012) (Figure 2A). Similarly, when cells were exposed to hypoxia condition for 24h, the PGC-1α gene expression decreased by 56.6% of control cells (1.047±0.10 vs. 0.455±0.12, P=0.002) (Figure 2B).
Figure 2.
Alterations in PGC-1α gene expression under oxidative stress conditions. PGC-1α gene expression in H4-IIE cells exposed to LPS (A) or hypoxia (B). Results from multiple experiments are shown as fold change over GAPDH levels. Data are presented as mean ± SE (n=6) and compared by compared by Student's t-test. * p<0.05 versus Control group.
Changes of PGC-1α gene expression and PGC-1α protein in rat liver
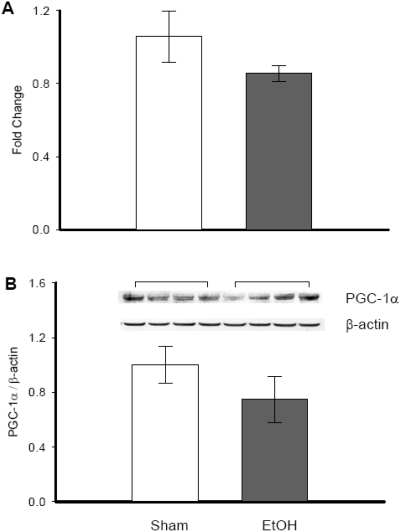
We further investigated the PGC-1α expression response to ethanol in vivo. Hepatic tissues extracted from ethanol treated rats showed slightly lower levels of PGC-1α gene (Figure 3A) and protein expression (Figure 3B) as compared to sham groups. However, the changes were not statistically significant.
Figure 3.
Alterations in PGC-1α gene and protein expression in ethanol exposed hepatic tissues. Hepatic tissues from rats exposed to ethanol (EtOH) or normal saline (Sham) were collected and both RNA and protein were isolated. A. RNA was used in real-time PCR and results are shown as fold change over GAPDH levels. B. Proteins were subjected to Western blotting using anti-PGC-1α antibody. Data are presented as mean ± SE (n=4) and compared by Student's t-test: no significant differences were observed.
Changes of LDH and AST in ethanol treated rats
Serum levels of LDH increased by 1.9 fold at 15h after ethanol administration (22.7±2.44 vs. 61.823±6.81, P= 0.006) as compared with sham-operated animals (Figure 4A). Likewise, tress serum levels of AST increased significantly by over 2.7 fold (32.7±1.02 vs.,d by 61.438±4.25, P=0.003) in the ethanol group (Figure 4B).
Figure 4.
Alterations in circulating levels of organ injury indicators following ethanol exposure: Plasma samples from normal saline (Sham) or ethanol (EtOH) treated rats were analyzed for LDH (A) and AST (B) using commercial assay kits. Data are presented as mean ± SE (n=4) and compared by Student's t-test. * p<0.05 versus sham group.
Changes in catalase expression in H4-IIE cells
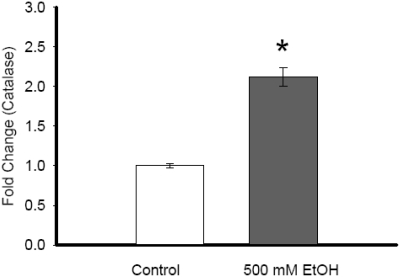
Catalase gene expression was significantly increased following 500 mM ethanol exposure in H4-IIE cells (1.003±0.04 vs. 2.12±0.18, P<0.001) as compared to control samples (Figure 5).
Figure 5.
Alterations in catalase gene expression due to ethanol exposure in hepatoma cells: Catalase gene expression of RNA extracted from control or ethanol (500 mM EtOH) was examined by real time PCR. Results are shown as fold change over GAPDH levels. Data are presented as mean ± SE (n=6) and compared by Student's t-test. * p<0.05 versus control group.
Discussion
In this study, we investigated the effect of ethanol on PGC-1α at gene expression and protein level in H4-IIE cells (in vitro) as well as in hepatic tissues (in vivo). Our data showed that the expression of PGC-1α decreased significantly in cells treated with 500 mM ethanol and that these levels were slightly decreased in the hepatic liver tissues. The discrepancy in the in vitro and in vivo data on the change in PGC-1α could be attributed to the absolute level of ethanol which can be reached to the liver as well as the time of collection of the samples following ethanol exposure in vivo. Interestingly, H4-IIE cells exposed to 500 mM ethanol showed significant increase in catalase, an enzyme that hydrolyzes peroxide to water, which is an indirect measure of ROS. This increase in amount of ROS is presumably caused by the ethanol-induced oxidative stress or ethanol oxidation. In fact, treatment of PC12 cells with catalase attenuated ethanol-induced oxidative stress [33]. PC12 cells treated with ethanol and catalase did not alter the ethanol concentration from that of ethanol alone suggesting that catalase did not catabolize ethanol in those cells. Thus the study suggested that catalase contributes to the decomposition of peroxide to water rather than the catabolism of ethanol per se. However, It has been reported that ethanol can serve as a substrate for catalase and that peroxide reacts with catalase to form a distinct complex termed compound I to form acetaldehyde and liberate catalase [34]. It is unclear from our data whether catalase contributes to the decomposition of peroxide to water or ethanol oxidation or both. It has also been shown that exposure to ethanol in brain microvascular endothelial cells (BMVEC) induced the expression of ethanol-metabolizing enzymes, cytochrome P450-2E1 and alcohol dehydrogenase, which paralleled enhanced generation of ROS [35]. Other oxidative stress conditions such as LPS or hypoxia are also known to increase ROS levels. Our study showed that PGC-1α gene expression was significantly impaired in either LPS or hypoxia conditions. It is plausible that LPS or hypoxia can induce mediators distinct from ROS which may affect PGC-1α expression. Further investigation is warranted to identify such mediators. Nevertheless, our results show that oxidative stress conditions such as acute alcohol consumption, LPS or hypoxia suppress PGC-1α expression in the liver, thereby presumably downregulate pertinent ROS-scavenging enzymes and enhance liver injury.
A substantial amount of evidence through human and animal studies indicates both chronic and acute alcohol abuse causes injury to liver as well as other organs and tissues [3,6,27,36], though the real factors that relate ethanol intake to onset of organ diseases are still not completely identified. Recent studies indicated that excessive ROS play an important role in alcohol induced cells or tissues injury. Li and colleague showed that PC12 cells exposed to ethanol for 24 hours produced increased levels of intracellular ROS [33]. Kupffer cells, the residential macrophages in the liver, are considered as an important source for ROS during ethanol exposure. It has been postulated that ethanol induced ROS generation could occur via CYP2E1, xanthine oxidase or nicotinamide dinucleotide phosphatase (NADPH) oxidase pathway [29]. LPS also increases the production of ROS via (NADPH) oxidase in macrophages. These ROS can contribute to activation of NFkB and the MAPK pathway [37] leading to the production of pro-inflammatory cytokines and cause liver injury.
The cause and effect of PGC-1α in ROS metabolism has not been clearly defined. However, there is strong evidence to show that the expression of ROS-detoxifying enzymes increases with over-expression of PGC-1α in 10T1/2 cells [20]. Furthermore, cells treated with viruses expressing siRNA directed against PGC-1α produced significantly reduced level of ROS-detoxifying enzymes, 1, SOD2 and GP×1 [20]. Rasbach and colleague showed that over-expression of PGC-1α in renal proximal tubular cells can promote recovery from mitochondrial dysfunction such as lipid peroxidation, ATP depletion or excess ROS generation [38]. Over expression of PGC-1α in vascular endothelial cells reduced accumulation of ROS and consequently downregulation of PGC-1α with siRNA reduced the expression of mitochondrial detoxifying proteins such as manganese SOD [22]. While we did not directly measure ROS levels, in an attempt to directly correlate ethanol induced ROS to PGC-1α, we studied the expression of catalase in H4-IIE cells treated with ethanol. Our studies showed that catalase expression was significantly increased in ethanol treated cells compared to control sample (Figure 5) suggesting a ‘causal relationship’ of ROS and ethanol. Further studies are warranted to confirm the actual cause and effect of PGC-1α and the suppression of ROS.
Kupffer cells are critical to alcohol induced liver injury. It has been shown that chronic exposure via gastric infusion in rats causes impaired gut permeability leading the release of LPS into the portal circulation. LPS recognition in Kupffer cells in the liver is mediated by the toll-like receptor 4 (TLR4). Upon activation of TLR4, IL-1 receptor associated kinases (IRAKs) and IkB kinases are recruited and activated leading to the stimulation of NFkB and cause the production of pro-inflammatory cytokines (TNF-α and IL-1), chemokines (IL-8, MCP-1) and TGFβ. These mediators act on other cells in the liver and cause liver damage. Chronic alcohol increases LPS-induced ERK1/2 activation which upregulate TNF-α expression via Egr-1 in rat Kupffer cells. In contrast, acute alcohol consumption inhibits TLR4 signaling in monocytes and macrophages which leads to decreased TNF-α production. Furthermore, acute alcohol exposure in human monocytes and macrophages suppresses LPS-induced IRAK phosphorylation. Nevertheless, it is evident that TLR4 and its downstream mediators are altered by alcohol and can contribute to alcohol induced liver injury.
In addition, breakdown of alcohol which occurs primarily in the hepatocytes causes oxygen deficits or hypoxia which in turn, impede the liver cells to produce ATP and thus contributes to cell death [39]. It has been postulated that high levels of ethanol induce microcirculatory disturbance and pericentral hypoxia which produce ROS in the mitochondria of hepatocytes [40]. Hypoxia also activates transcription via a mitochondria-dependent signaling process involving increased ROS in HepSB cells [41]. Thus, the alcohol induced liver injury could possibly be caused by multiple factors such as hypoxia, oxidative stress and the activation of pro-inflammatory cytokines.
In conclusion, our study suggests that ethanol exposure attenuates PGC-1α thereby possibly inhibits the expression of pertinent ROS-scavenging enzymes and enhances liver injury.
Acknowledgments
This work was supported by NIH grant R01 GM053008 (P. Wang). The authors thank Marrisa Steinberg for her excellent technical assistance and all the members of the surgical research team for insightful suggestions and discussions.
References
- 1.Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol. 2007;13:4967–73. doi: 10.3748/wjg.v13.i37.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H, Takei Y, Hirose M, Shimizu H, Miyazaki A, Brenner DA, Sato N, Thurman RG. Role of Kupffer cells and gutderived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;(Suppl 15):D20–5. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 3.Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–11. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 4.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 5.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–51. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 6.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257–72. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 7.Zima T, Kalousova M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]
- 8.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–63. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–7. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 11.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 12.Wan YJ, Morimoto M, Thurman RG, Bojes HK, French SW. Expression of the peroxisome proliferator-activated receptor gene is decreased in experimental alcoholic liver disease. Life Sci. 1995;56:307–17. doi: 10.1016/0024-3205(94)00953-8. [DOI] [PubMed] [Google Scholar]
- 13.Galli A, Pinaire J, Fischer M, Dorris R, Crabb DW. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor alpha is inhibited by ethanol metabolism A novel mechanism for the development of ethanol-induced fatty liver. J Biol Chem. 2001;276:68–75. doi: 10.1074/jbc.M008791200. [DOI] [PubMed] [Google Scholar]
- 14.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 15.Jokelainen K, Reinke LA, Nanji AA. Nfkappab activation is associated with free radical generation and endotoxemia and precedes pathological liver injury in experimental alcoholic liver disease. Cytokine. 2001;16:36–9. doi: 10.1006/cyto.2001.0930. [DOI] [PubMed] [Google Scholar]
- 16.Wang XD, Liu C, Chung J, Stickel F, Seitz HK, Russell RM. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744–50. doi: 10.1002/hep.510280321. [DOI] [PubMed] [Google Scholar]
- 17.Boelsterli UA, Bedoucha M. Toxicological consequences of altered peroxisome proliferator-activated receptor gamma (PPARgamma) expression in the liver: insights from models of obesity and type 2 diabetes. Biochem Pharmacol. 2002;63:1–10. doi: 10.1016/s0006-2952(01)00817-6. [DOI] [PubMed] [Google Scholar]
- 18.Greiffenstein P, Mathis KW, Stouwe CV, Molina PE. Alcohol binge before trauma/hemorrhage impairs integrity of host defense mechanisms during recovery. Alcohol Clin Exp Res. 2007;31:704–15. doi: 10.1111/j.1530-0277.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 19.Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KL, Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther. 2003;306:846–54. doi: 10.1124/jpet.102.047217. [DOI] [PubMed] [Google Scholar]
- 20.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 21.St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators lalpha and Ibeta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 22.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–73. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Houten SM, Auwerx J. PGC-1alpha: turbocharging mitochondria. Cell. 2004;119:5–7. doi: 10.1016/j.cell.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 25.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 26.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 27.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–26. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]
- 28.Dicker E, Cederbaum AL. Increased NADH-dependent production of reactive oxygen intermediates by microsomes after chronic ethanol consumption: comparisons with NADPH. Arch Biochem Biophys. 1992;293:274–80. doi: 10.1016/0003-9861(92)90395-d. [DOI] [PubMed] [Google Scholar]
- 29.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–56. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista AP. Acute ethanol binge followed by withdrawal regulates production of reactive oxygen species and cytokine-induced neutrophil chemoattractant and liver injury during reperfusion after hepatic ischemia. Antioxid Redox Signal. 2002;4:721–31. doi: 10.1089/152308602760598864. [DOI] [PubMed] [Google Scholar]
- 31.Phelan H, Stahls P, Hunt J, Bagby GJ, Molina PE. Impact of alcohol intoxication on hemodynamic, metabolic, and cytokine responses to hemorrhagic shock. J Trauma. 2002;52:675–82. doi: 10.1097/00005373-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Zhou M, Ba ZF, Cioffi WG, Chaudry IH. Up-regulation of a novel potent vasodilatory peptide adrenomedullin during polymicrobial sepsis. Shock. 1998;10:118–22. doi: 10.1097/00024382-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Walker DW, King MA. Peroxide mediates ethanol-induced cytotoxicity in PC12 cells. Free Radic Biol Med. 2001;30:389–92. doi: 10.1016/s0891-5849(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 34.Aragon CM, Stotland LM, Amit Z. Studies on ethanol-brain catalase interaction: evidence for central ethanol oxidation. Alcohol Clin Exp Res. 1991;15:165–9. doi: 10.1111/j.1530-0277.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 35.Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–32. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- 36.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–71S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 37.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–9. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 38.Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–9. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham CC, Van Horn CG. Energy availability and alcohol-related liver pathology. Alcohol Res Health. 2003;27:291–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N. Central role of mitochondria in metabolic regulation of liver pathophysiology. J Gastroenterol Hepatol. 2007;22(1):Sl–6. doi: 10.1111/j.1440-1746.2007.04963.x. [DOI] [PubMed] [Google Scholar]
- 41.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]