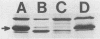Abstract
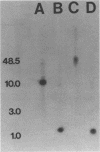
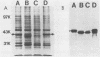
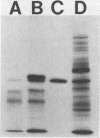
The major outer membrane protein (P2) of Haemophilus influenzae type b (Hib) with an apparent molecular weight of 37,000 to 40,000 has been previously shown to function as a porin and also as a target for antibodies protective against experimental Hib disease. The gene encoding the Hib P2 protein was cloned by using a shuttle vector capable of replication in both Escherichia coli and H. influenzae. The amino acid sequence of the amino terminus of the Hib P2 protein was determined and used to design an oligonucleotide probe corresponding to the first 20 amino acids of this protein. This oligonucleotide probe was used to identify Hib chromosomal DNA fragments containing the Hib P2 gene. These DNA fragments were ligated into the plasmid vector pGJB103 and then used to transform a rec-1 mutant of H. influenzae Rd. Recombinant clones expressing the Hib P2 protein were identified in a colony blot-radioimmunoassay by using a monoclonal antibody specific for a surface epitope of the Hib P2 protein. The gene encoding this Hib protein was present on a 10-kilobase Hib DNA insert in the recombinant plasmid. Transformation experiments involving the recombinant plasmid suggested that unregulated synthesis of Hib P2 is a lethal event in E. coli. The recombinant Hib P2 protein was exposed on the surface of the recombinant H. influenzae strain. This recombinant strain was used to develop a system for detecting polyclonal serum antibodies directed against surface determinants of the Hib P2 protein. The availability of the gene encoding the Hib P2 protein should facilitate investigation of both the immunogenicity and the structure-function relationship(s) of this major outer membrane protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bricker J., Mulks M. H., Plaut A. G., Moxon E. R., Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1983 May;80(9):2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Smith A. L. A major outer-membrane protein functions as a porin in Haemophilus influenzae. J Gen Microbiol. 1987 May;133(5):1273–1277. doi: 10.1099/00221287-133-5-1273. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochi S. L., Broome C. V. Vaccine prevention of Haemophilus influenzae type b disease: past, present and future. Pediatr Infect Dis. 1986 Jan-Feb;5(1):12–19. doi: 10.1097/00006454-198601000-00003. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Danner D. B., Pifer M. L. Plasmid cloning vectors resistant to ampicillin and tetracycline which can replicate in both E. coli and Haemophilus cells. Gene. 1982 Apr;18(1):101–105. doi: 10.1016/0378-1119(82)90062-2. [DOI] [PubMed] [Google Scholar]
- Deich R. A., Metcalf B. J., Finn C. W., Farley J. E., Green B. A. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J Bacteriol. 1988 Feb;170(2):489–498. doi: 10.1128/jb.170.2.489-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub R. Mutants of the mini-F plasmid pML31 thermosensitive in replication. J Bacteriol. 1979 May;138(2):559–566. doi: 10.1128/jb.138.2.559-566.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales F. R., Leachman S., Norgard M. V., Radolf J. D., McCracken G. H., Jr, Evans C., Hansen E. J. Cloning and expression in Escherichia coli of the gene encoding the heat-modifiable major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2993–3000. doi: 10.1128/iai.55.12.2993-3000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M. E., Blake M. S., Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8135–8139. doi: 10.1073/pnas.84.22.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Green B. A., Quinn-Dey T., Zlotnick G. W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987 Dec;55(12):2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Hansen E. J. Coprecipitation of lipopolysaccharide and the 39,000-molecular-weight major outer membrane protein of Haemophilus influenzae type b by lipopolysaccharide-directed monoclonal antibody. Infect Immun. 1985 Sep;49(3):819–827. doi: 10.1128/iai.49.3.819-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Patrick C. C., Hermanstorfer L., McCracken G. H., Jr, Hansen E. J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987 Mar;55(3):513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Hart D. A., McGehee J. L., Toews G. B. Immune enhancement of pulmonary clearance of nontypable Haemophilus influenzae. Infect Immun. 1988 Jan;56(1):182–190. doi: 10.1128/iai.56.1.182-190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. L., Loftus T. A., Hansen E. J. Cloning and surface expression in Escherichia coli of a structural gene encoding a surface protein of Haemophilus influenzae type b. Infect Immun. 1985 Oct;50(1):236–242. doi: 10.1128/iai.50.1.236-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Hansen E. J. Antigenic and phenotypic variations of Haemophilus influenzae type b lipopolysaccharide and their relationship to virulence. Infect Immun. 1986 Jan;51(1):69–79. doi: 10.1128/iai.51.1.69-79.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Hirth P., DeWilde M., Harford N., Lecocq J. P. Cell-free synthesis of enterotoxin of E. coli from a cloned gene. Nature. 1980 Apr 3;284(5755):473–474. doi: 10.1038/284473a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Woodin K. A. Cross-reactivity of surface-exposed epitopes of outer membrane antigens of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2977–2983. doi: 10.1128/iai.55.12.2977-2983.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Grass S. Purification, cloning, and sequence of outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1988 Sep;56(9):2235–2242. doi: 10.1128/iai.56.9.2235-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Campagnari A. A., Nelson M. B., Apicella M. A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986 Dec;54(3):774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Nelson M. B., Dudas K. C., Mylotte J. M., Apicella M. A. Identification of a specific epitope of Haemophilus influenzae on a 16,600-dalton outer membrane protein. J Infect Dis. 1985 Dec;152(6):1300–1307. doi: 10.1093/infdis/152.6.1300. [DOI] [PubMed] [Google Scholar]
- Nelson M. B., Apicella M. A., Murphy T. F., Vankeulen H., Spotila L. D., Rekosh D. Cloning and sequencing of Haemophilus influenzae outer membrane protein P6. Infect Immun. 1988 Jan;56(1):128–134. doi: 10.1128/iai.56.1.128-134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C. C., Kimura A., Jackson M. A., Hermanstorfer L., Hood A., McCracken G. H., Jr, Hansen E. J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun. 1987 Dec;55(12):2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenep J. L., Munson R. S., Jr, Barenkamp S. J., Granoff D. M. Further studies of the role of noncapsular antibody in protection against experimental Haemophilus influenzae type b bacteremia. Infect Immun. 1983 Oct;42(1):257–263. doi: 10.1128/iai.42.1.257-263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Effect of glycerol on plasmid transfer in genetically competent Haemophilus influenzae. Mol Gen Genet. 1986 May;203(2):296–299. doi: 10.1007/BF00333969. [DOI] [PubMed] [Google Scholar]
- Thomas W. R., Rossi A. A. Molecular cloning of DNA coding for outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1986 Jun;52(3):812–817. doi: 10.1128/iai.52.3.812-817.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon V., Laprade R., Coulton J. W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986 Sep 25;861(1):74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]