Abstract
Competition has long been proposed as an important force in structuring mammalian communities. Although early work recognized that competition has a phylogenetic dimension, only with recent increases in the availability of phylogenies have true phylogenetic investigations of mammalian community structure become possible. We test whether the phylogenetic structure of 142 assemblages from three mammalian clades (New World monkeys, North American ground squirrels and Australasian possums) shows the imprint of competition. The full set of assemblages display a highly significant tendency for members to be more distantly related than expected by chance (phylogenetic overdispersion). The overdispersion is also significant within two of the clades (monkeys and squirrels) separately. This is the first demonstration of widespread overdispersion in mammal assemblages and implies an important role for either competition between close relatives where traits are conserved, habitat filtering where distant relatives share convergent traits, or both.
Keywords: net relatedness index, nearest taxon index, possum, squirrel, monkey, community phylogenetics
1. Introduction
Interspecific competition has been shown to occur frequently in nature (Connell 1983; Schoener 1983) and has been suggested as an important force in structuring mammalian communities for many years. This theory assumes that species that are too ecologically similar will be unable to coexist due to competitive exclusion of the inferior competitor (Hutchinson 1959), so communities should contain only species that are sufficiently different to coexist. Because ecological similarity is often highest among closely related species that share traits from a recent common ancestor (Harvey & Pagel 1991), competition must have a phylogenetic dimension. This has long been recognized: Darwin (1859) proposed that species in the same genus would be more likely to compete than those in different genera. However, without access to phylogenies, early work on mammalian community structure could only use taxonomy as a surrogate for phylogeny by looking at, for instance, species-to-genus ratios (Elton 1946).
If competition is important in structuring mammalian communities, few species per genus are expected to coexist in each community (Elton 1946; table 1). Early studies often found such a pattern; however, these ratios depend strongly on the number of species involved and, once this was taken into account, later work revealed that many communities contained more species per genus than expected (Jarvinen 1982). This result suggests that other factors such as habitat filtering, where only ecologically similar species that share traits enabling them to survive in a locality can coexist, may also be shaping community assembly. Such factors will also have a phylogenetic dimension (Harvey & Pagel 1991).
Table 1.
Schematic demonstrating how habitat filtering and competition affect patterns in the distribution of community members across phylogenies (black dots), species-to-genus ratios and the phylogenetic structure of communities. NRI, net relatedness index; NTI, nearest taxon index.
| process | habitat filtering: species share traits that allow them to exist in a particular environment | random: neither process strongly effects community assembly nor multiple factors working in opposing directions | competition: only species that are not too ecologically similar are able to coexist |
| traits | conserved: if traits are convergent the patterns are similar to those shown for competition | either conserved or convergent | conserved: if traits are convergent the patterns are similar to those shown for random communities |
| distribution of community species on phylogeny | 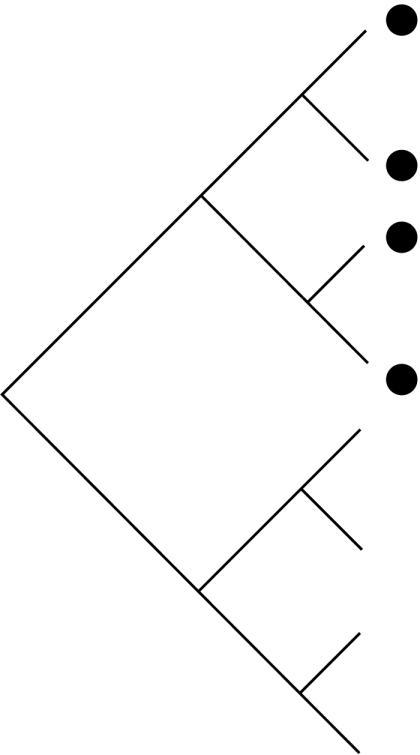 |
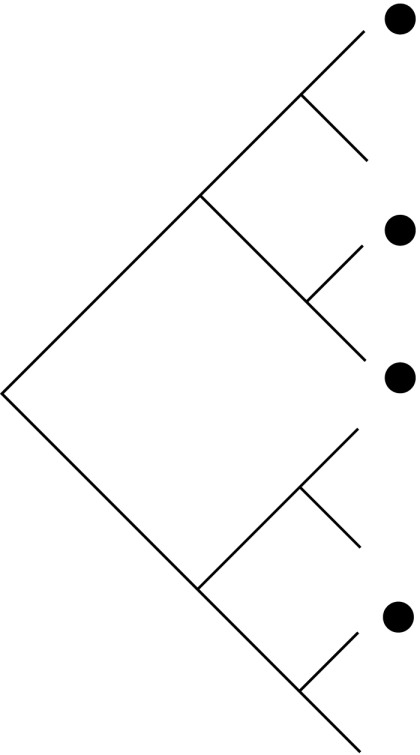 |
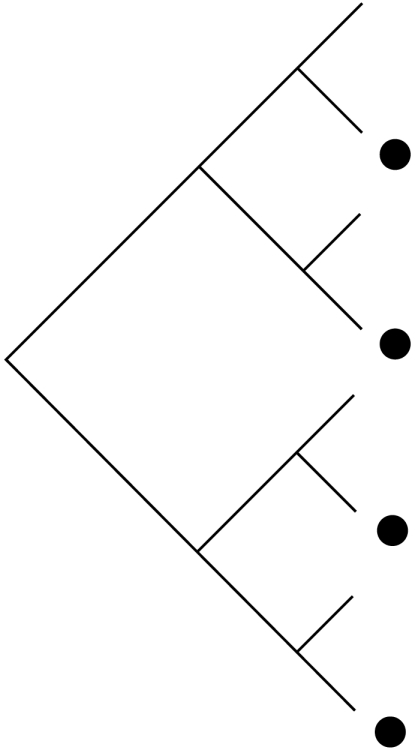 |
| species : genus ratio | more species per genus than expected by chance | no more or less species per genus than expected by chance | fewer species per genus than expected by chance |
| phylogenetic structure | phylogenetic clustering (positive NRI and NTI scores) | random phylogenetic structure (NRI and NTI scores not significantly different from zero) | phylogenetic overdispersion (negative NRI and NTI scores) |
With recent increases in the availability of phylogenies we can now use phylogeny, rather than taxonomy, to look at community structure. Table 1 outlines the taxonomic and phylogenetic patterns expected under different assembly rules. If competition affects community membership, then species in a community will be more distantly related than expected by chance (phylogenetic overdispersion; Webb et al. 2002). If, conversely, community membership is determined by habitat filtering, the species within a community will be more closely related than expected by chance (phylogenetic clustering; Webb et al. 2002). Finally, if community assembly is not strongly influenced by phylogeny, or if multiple factors oppose and nullify each other, community lists will be randomly assembled with respect to phylogeny (Helmus et al. 2007).
These ideas form the basis of ‘community phylogenetic’ methods (Webb et al. 2002), which compare the phylogenetic position of community members with those of non-members from a regional source pool. Unfortunately, the patterns are not always easy to interpret: phylogenetic overdispersion may also result from convergence of distantly related species (where traits are convergent rather than conserved; Cavender-Bares et al. 2004; Kraft et al. 2007). Likewise, phylogenetic clustering may also be due to historical or biogeographical factors, in situ speciation, or limits on species dispersal, which prevent species from leaving their ancestral ranges.
Here we aim to determine whether the predictions of competition as a powerful structuring force in mammalian communities are met by community phylogenies. Previous studies of phylogenetic structure of communities have focused on plants and microbes (e.g. Webb 2000; Cavender-Bares et al. 2004; Horner-Devine & Bohannon 2006) but to our knowledge only one other study has looked at mammalian communities. Cardillo et al. (2008) performed a global-scale analysis of the phylogenetic structure of island mammal assemblages over broad taxonomic groupings. Here we instead investigate patterns in three geographically restricted and moderately diverse mammalian clades: New World monkeys (Platyrrhini); Australasian possums (Phalangeriformes); and North American ground squirrels (Marmotini). This narrower phylogenetic focus should increase the likelihood of competition among the species (Darwin 1859) as they should require more similar resources. In addition, the clades exhibit a range of life histories and live in a variety of habitats allowing us to test the generality of any patterns observed.
We use two measures of assemblage phylogenetic structure (see below and table 1): Webb's (Webb 2000; Webb et al. 2002) net relatedness index (NRI) and nearest taxon index (NTI), to investigate the phylogenetic structure of multiple assemblages for each clade and focus on general patterns rather than on those of individual assemblages (as done in most previous studies). Using this simple pooled approach, we find a general pattern of phylogenetic overdispersion across all of our clades combined, which is significant within two of the clades (squirrels and monkeys) separately, suggesting that competition does have a role in mammalian community assembly.
2. Material and Methods
(a) Data
(i) Species assemblages and species pools
There has been much debate over the exact definition of the term community (Underwood 1986) so to avoid confusion we instead refer to assemblages, defined as a group of species that co-occur and potentially interact (Begon et al. 1996). Assemblage membership was determined using mammal species checklists for a particular area compiled by J.R. These checklists were selected from a database of mammal assemblages including 376 georeferenced localities varying in size from 10 to 440 000 km2 (we return to the differences among the sizes of our localities below) and are distributed worldwide. The lists came from several published sources reporting inventories of species observed inside these areas. Only localities with inventories considered to be ‘complete’ by the original source, or that may be assumed to be complete from the information provided therein, were included in the database. Exotic species were excluded and for these analyses taxonomy was standardized following Wilson & Reeder (1993) since both the supertree of Bininda-Emonds et al. (2007) and the geographical range maps (see below) use this taxonomy. None of our clades contain recently extinct species. Other examples of the application of this database may be seen in Rodríguez et al. (2006) and Hortal et al. (2008). In total, we obtained data for 34 monkey, 13 possum and 95 squirrel assemblages, details of which can be found in appendices S1 and S2 in electronic supplementary material. These particular clades were chosen as they form part of an ongoing PhD thesis.
The species pool for each assemblage was calculated by overlaying polygon geographical range maps in ArcGIS from Sechrest (2003) and extracting all species occurring within the assemblage locality and up to a threshold distance of 500 km outside the locality boundary. Community phylogenetic studies can be strongly dependent on the spatial scale of the species pool (Swenson et al. 2006), so we repeated the analyses using threshold distances of 100, 250 and 1000 km when defining species pools. Assemblage phylogenetic structure is calculated relative to that of the species pool so that it cannot be calculated if the assemblage constitutes the entire species pool. This happens more frequently with smaller thresholds: out of the 142 assemblages, 67 represented the complete species pool when 100 km pools were used; 45 when 250 km pools were used; 29 when 500 km pools were used; and 26 when 1000 km pools were used. Since the 1000 km pool analyses contained only three extra assemblages, and at this scale it is probable that some of the species included in the pool could not feasibly be members of the assemblage due to the geographical distances involved, we report results from the 500 km species pool analyses. However, pool size made no qualitative difference to the results (appendix S3, table S1 in electronic supplementary material).
(ii) Phylogeny
The phylogenetic topology came from Bininda-Emonds et al. (2007). Many of the node ages in that paper were affected by a software bug, so we used corrected node ages (O. Bininda-Emonds 2008, personal communication). NRI and NTI (see below) require a well-resolved phylogeny since polytomies reduce their power to detect phylogenetic structure. Therefore, we added further resolution within squirrel genera using the subgenus assignments of Wilson & Reeder (2005), and within monkey genera (e.g. Aotus) using a more resolved primate supertree (Vos 2006). In the Bininda-Emonds et al. (2007) supertree, the relationship among the genera Cynomys, Marmota and Spermophilus is unresolved. We created three new phylogenies, one for each possible resolution of the polytomy and analysed each in turn. The topology used had no qualitative influence on the results (appendix S3, table S2 in electronic supplementary material), so we only report the results for the Cynomys out-group tree. Each of the phylogenies used was completely resolved and they are available in appendix S4, electronic supplementary material. Within the supertree of Bininda-Emonds et al. (2007), 30.1% of the monkey node dates, 45.5% of the possum node dates and 11.2% of the squirrel node dates were based on molecular estimates; the rest of the node dates were interpolated.
(b) Analyses
All data analysis (described in detail below) was carried out using R v. 2.5.1 (R Development Core Team 2007) and we analysed the three mammalian clades separately.
(iii) Measuring phylogenetic assemblage structure
We determined NRI and NTI values (Webb 2000; Webb et al. 2002; see appendix 3, figure S1 in electronic supplementary material for example calculations) for each assemblage that had more than one species and did not constitute the complete source pool. NRI and NTI are both measures of the phylogenetic distance between taxa in an assemblage, where phylogenetic distance is defined as the sum of all intervening branch lengths between two taxa. However, they reflect phylogenetic structure in different parts of the phylogeny. NRI is based on the mean phylogenetic distance (MPD) of an assemblage, i.e. the MPD between all possible pairs of taxa within the assemblage (), and significant values reflect clustering or overdispersion across the whole of the pool phylogeny. NTI, on the other hand, is based on the mean nearest neighbour distance (MNND), i.e. the mean distance between each of n taxa (where n is the number of taxa in the assemblage) within the assemblage and its nearest neighbour in the assemblage phylogeny (). NTI is therefore most sensitive to clustering or overdispersion near the tips of the pool phylogeny. To allow comparisons between multiple assemblages, these MPD and MNND values are then standardized by (i) subtracting the mean MPD/MNND expected for n taxa drawn at random from the species pool using 10 000 iterations (/) and then (ii) dividing by the standard deviation of the MPD/MNND from these 10 000 randomly drawn pseudo-assemblages (s(MPDn)/s(MNNDn)). Both values are then multiplied by −1 so that clustered NRI and NTI values are positive and overdispersed values are negative, which is more intuitive than the reverse. Thus, NRI and NTI are calculated as follows:
This procedure should mean that NTI and NRI are approximately normally distributed; however, previous studies have shown that NRI is generally biased towards detecting overdispersion owing to the branching structure of phylogenies (Kembel & Hubbell 2006; Swenson et al. 2006). Therefore, to test whether an individual assemblage was significantly clustered or overdispersed we compared MPD and MNND values for the real assemblage with those from the 10 000 randomly generated pseudo-assemblages (with n species drawn at random from the assemblage's species pool) used to calculate NRI and NTI. A particular assemblage was considered significantly clustered if less than 250 (2.5%) of these random assemblages had a larger MPD/MNND value than that of the assemblage or overdispersed if less than 250 (2.5%) had a lower MPD/MNND value than that of the assemblage.
(iv) Pooled NRI/NTI analyses
Null model choice is vital to interpreting the results of NRI and NTI (Kembel & Hubbell 2006). We were therefore unable to test for trends across all assemblages using raw NRI values because NRI values tend to be negatively skewed, making the null expectation of any test uncertain. Therefore, we devised a novel and simple non-parametric method to analyse our pooled data.
For each assemblage, we first calculated NRI for 10 000 randomly generated pseudo-assemblages (with n species drawn at random from the assemblage's species pool). We then determined the centile of the observed NRI (which we term NRI%) within this distribution; if the observed value was tied with multiple random assemblages, the centile of the median of these tied values was used. If assemblage members are random picks from the source pool, the expected centile is 50 (i.e. the median of the null distribution). Thus, the expected median of the NRI% values, across the set of assemblages, was also 50. We therefore tested whether the median NRI% differed from 50 using Wilcoxon tests. Since this result used assemblages from all three clades, we also used Kruskal–Wallis rank sum tests to determine whether the different clades had significantly different NRI% values from each other.
NTI is expected to be approximately normally distributed with a mean of zero so we used t-tests to determine whether the mean of the distribution differed from zero demonstrating a general trend towards either clustering or overdispersion. We then used analysis of variance (ANOVA) to determine whether the different clades had significantly different NTI values from each other.
(v) Methodological bias
As mentioned above, our assemblage localities ranged in size from 10 to 440 000 km2 and also contained different numbers of species. If NRI% or NTI values are affected by these factors, they may bias our results. We therefore used Spearman's rank correlation tests to determine whether NRI% or NTI values were correlated with the ln-area of the assemblage locality (km2), ln-assemblage species richness, ln-species pool species richness or assemblage: pool species richness ratio (calculated as assemblage species richness/species pool species richness). The results of these analyses can be found in appendix S3, table S3, figure S2 in the electronic supplementary material.
3. Results
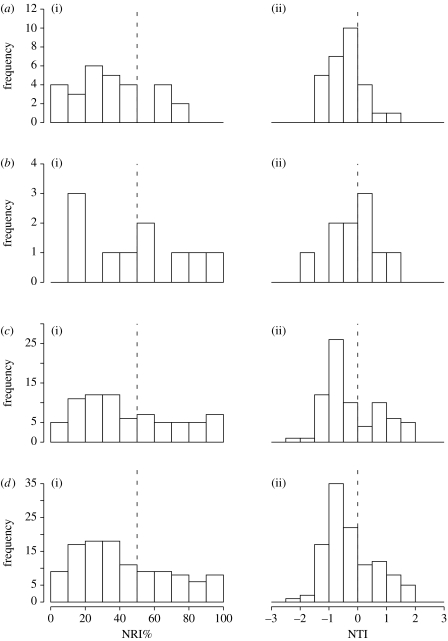
The Wilcoxon tests showed that the median NRI% (NRI centiles) differed significantly from 50 for all assemblages combined (p=0.004) and for monkeys (p=0.001) but not for possums (p=0.999) or squirrels (p=0.082; table 2; figure 1).
Table 2.
Results of Wilcoxon tests investigating whether the distribution of NRI% values has medians significantly different from 50, and of t-tests investigating whether the distribution of NTI values have means significantly different from zero, for all clades and each clade separately. Key: monkeys, New World monkeys (Platyrrhini); possums, Phalangeriformes; squirrels, North American ground squirrels (Marmotini). n, Number of communities. *p<0.05; **p<0.01.
| clade | n | median NRI% (first and third quartiles) | V | mean NTI±s.e. | t |
|---|---|---|---|---|---|
| monkeys | 28 | 31.2 (19.6, 46.9) | 67** | −0.401±0.118 | −3.40** |
| possums | 10 | 49.5 (20.5, 73.1) | 27 | −0.055±0.261 | −0.21 |
| squirrels | 75 | 37.1 (25.0, 64.0) | 1095 | −0.222±0.108 | −2.06* |
| all | 113 | 36.1 (20.8, 63.9) | 2201** | −0.251±0.081 | −3.12** |
Figure 1.
((a–d) i) Distributions of NRI% values and ((a–d) ii) NTI values for each clade separately and all clades combined. (a) New World monkeys (Platyrrhini), (b) Possums (Phalangeriformes), (c) North American ground squirrels (Marmotini) and (d) all clades combined. In the NRI% plots, the dashed line represents the fiftieth centile (median). In the NTI plots, the dashed line is where NTI equals zero.
The t-tests showed that the mean NTI values differed significantly from zero for all assemblages combined (p=0.002) for monkeys (p=0.002) and for squirrels (p=0.043), but not for possums (p=0.837; table 2; figure 1).
The NRI% and NTI values for possums were closer to the null expectation but there were no significant differences among clades in either NRI% or NTI (NRI%: Kruskal–Wallis Χ22=2.91, p=0.23; NTI: F2,110=0.73, p=0.48).
When the assemblages were considered individually, only 6 squirrel assemblages out of 75 (Coram Biosphere Reserve, MT: NRI=−1.71, p=0.006; Dinosaur NM: NTI=−1.67, p=0.046; Guadalupe Mountains: NTI=−1.39, p=0.04; Yellowstone NP (USA): NRI=−1.10, p=0.037; Zion: NRI=−1.23, p=0.006, NTI=−2.06, p=0.010) showed significant overdispersion. None of the individual monkey or possum assemblages were significantly overdispersed. No individual assemblages were significantly clustered. NRI and NTI values for individual localities are given in appendix S1 in electronic supplementary material.
4. Discussion
Our results suggest a consistent tendency for mammalian assemblages to be more phylogenetically overdispersed than expected by chance, and that this tendency is only detectable by pooling many assemblages. This pattern is seen both across the whole phylogeny (NRI/NRI results) and at the tips (NTI/NTI results). Additionally, the three clades analysed do not have significantly different NRI% or NTI values suggesting that this could be a general, rather than a clade-specific mammalian pattern.
The traditional interpretation of phylogenetic overdispersion is that competition among ecologically similar close relatives has led to exclusion of the inferior competitors and hence an assemblage with more distantly related species than expected. This fits with the predictions of earlier non-phylogenetic studies looking at how competition structures mammalian communities and with the results of Houle (1997), who found that primates that were phylogenetically ‘too close’ did not coexist. However, if traits allowing a species to exist in an area have evolved convergently in more distant relatives, then habitat filtering could also cause overdispersion (Cavender-Bares et al. 2004; Kraft et al. 2007). Determining whether competition or habitat filtering is more likely would require an analysis integrating data on the traits mediating competition and resource use. For example, Davies et al. (2007) demonstrated a role for competition in structuring carnivoran assemblages, by analysing geographical range overlap together with differences in traits implicated in interspecific competition (body size and tooth morphology). In the absence of information about which traits in our groups most strongly influence competition, it is only possible to make weak inferences from ancillary information. Habitat filtering may be more likely in squirrels, because convergence is hypothesized in a range of skeletal traits (Roth 1996), and because our assemblages come from a mixture of grassland, desert and woodland habitat types. Furthermore, the species pools for squirrels tended to be relatively large (median=15 species, first quartile=5.5 and third quartile=22), increasing power to detect habitat filtering but decreasing power to detect competition (Kraft et al. 2007). Competition may be more likely in monkeys: they had smaller species pools (median=8 species, first quartile=5.75 and third quartile=11.5) and all the species come from broadly similar habitat types, perhaps reducing the scope for habitat filtering. Possum assemblages may be generally assembled at random with respect to phylogeny, perhaps because the traits involved in habitat filtering and competition in this group are independent of phylogeny, or because both mechanisms are acting and have cancelled each other out (e.g. Helmus et al. 2007). However, these results are based on only 10 assemblages, so may not permit any robust conclusions.
Interestingly, few individual assemblages had significantly overdispersed NRI or NTI values. This could be due to low statistical power, since both our assemblages and species pools tended to be small, and most assemblages were either too small or too large with respect to the pool for maximum power (Kraft et al. 2007). In addition, competition does not always lead to competitive exclusion. Some species may be temporally or spatially segregated within the same habitat. Other species show behavioural plasticity, which allows similar species to coexist (Houle 1997; Lovette & Hochachka 2006), or rapidly evolve different ecotypes (Harmon et al. 2003). Finally, our results focus on how competition between close relatives may limit species distributions in some mammalian clades. However, other factors also influence where species occur, such as geographical boundaries, limits on dispersal, differential extinction (human mediated or otherwise), the distribution of resources (e.g. food and shelter) and interactions with species from different clades (e.g. predation). Although the narrow taxonomic focus of our approach should increase the likelihood of detecting competition in our clades (Darwin 1859), distant relatives may also compete. For example, in the neotropics, frugivorous bats compete with birds (Palmeirim 1989) and potentially with monkeys as well.
Our assemblage lists come from species checklists, which may be incomplete. This incompleteness could only undermine our results if, for some reason, the omissions caused the species on the lists to appear phylogenetically overdispersed. An alternative approach would be to use species range map overlap to determine the assemblage membership (e.g. Davies et al. 2007). However, that approach is also problematic as maps tend to overestimate species ranges and hence species overlap (Hurlbert & Jetz 2007). Species checklists, while imperfect, are much more likely to capture sets of interacting species. Here we use range maps only to delimit source pools, so any errors will affect only the pools; and we show that varying the threshold for inclusion in the source pool has no qualitative impact on the results. Our NRI results also appear to be influenced by the size of the species pool and our NTI results by the ratio of the number of species in the assemblage to the number of species in the pool (appendix S3, table S3 in electronic supplementary material). Since where NRI was affected by these factors, NTI was not, and vice versa, we believe that the general pattern of overdispersion in our assemblages was not merely due to methodological bias. However, these factors should be considered in future studies.
Our results suggest that species distributions are influenced by those of other closely related species. Since species geographical range maps do not reflect the local heterogeneity in distributions that may be caused by such interactions, our knowledge of where species actually occur may be unreliable. Previous authors have recognized this and its implications for biodiversity research and conservation (Hurlbert & Jetz 2007). Likewise, the current trend for mapping how geographical ranges will shift under particular climate change scenarios may underestimate how species will be affected if these among-species interactions are ignored. Such interactions may make it much harder to predict how species will respond to their rapidly changing environment.
Acknowledgments
We thank Olaf Bininda-Emonds, Marcel Cardillo, Gavin Thomas, Joaquin Hortal, Susanne Fritz, Lynsey McInnes, Tom Ezard, Rich Grenyer, Sarah Holbrook, Kate Jones, Owen Jones, Alex Pigot, Nicola Toomey, David Ackerley and three anonymous referees. N.C. was funded by NERC (NER/S/A/2005/13577). J.R. was supported by the Spanish MEC project (CGL2006-13532-C03/BTE).
Supplementary Material
References
- Begon M, Harper J.L, Townsend C.R. Blackwell Science; Oxford, UK: 1996. Ecology of individuals, populations and communities. [Google Scholar]
- Bininda-Emonds O.R.P, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. doi:10.1038/nature05634 [DOI] [PubMed] [Google Scholar]
- Cardillo M, Gittleman J.L, Purvis A. Global patterns in the phylogenetic structure of island mammal assemblages. Proc. R. Soc. B. 2008;275:1549–1556. doi: 10.1098/rspb.2008.0262. doi:10.1098/rspb.2008.0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender-Bares J, Ackerly D.D, Baum D.A, Bazzaz F.A. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 2004;163:823–843. doi: 10.1086/386375. doi:10.1086/386375 [DOI] [PubMed] [Google Scholar]
- Connell J.H. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am. Nat. 1983;122:661–696. doi:10.1086/284165 [Google Scholar]
- Darwin C. John Murray; London, UK: 1859. On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. [PMC free article] [PubMed] [Google Scholar]
- Davies J.T, Meiri S, Barraclough T.G, Gittleman J.L. Species co-existence and character divergence across carnivores. Ecol. Lett. 2007;10:146–152. doi: 10.1111/j.1461-0248.2006.01005.x. doi:10.1111/j.1461-0248.2006.01005.x [DOI] [PubMed] [Google Scholar]
- Elton C.S. Competition and the structure of animal communities. J. Anim. Ecol. 1946;15:54–68. doi:10.2307/1625 [Google Scholar]
- Harmon L.J, Schulte J.A, II, Larson A, Losos J.B. Tempo and mode of evolutionary radiation in iguanian lizards. Science. 2003;301:961–964. doi: 10.1126/science.1084786. doi:10.1126/science.1084786 [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford, UK: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Helmus M.R, Savage K, Diebel M.W, Maxted J.T, Ives A.R. Separating the determinants of phylogenetic community structure. Ecol. Lett. 2007;10:917–925. doi: 10.1111/j.1461-0248.2007.01083.x. doi:10.1111/j.1461-0248.2007.01083.x [DOI] [PubMed] [Google Scholar]
- Horner-Devine M.C, Bohannon B.J.M. Phylogenetic clustering and overdispersion in bacterial communities. Ecology. 2006;87:S100–S108. doi: 10.1890/0012-9658(2006)87[100:pcaoib]2.0.co;2. doi:10.1890/0012-9658(2006)87[100:PCAOIB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hortal J, Rodríguez J, Nieto-Díaz M, Lobo J.M. Regional and environmental effects on the species richness of mammal assemblages. J. Biogeogr. 2008;35:1202–1214. doi:10.1111/j.1365-2699.2007.01850.x [Google Scholar]
- Houle A. The role of phylogeny and behavioral competition in the evolution of coexistence among primates. Can. J. Zool. -Revue Canadienne De Zoologie. 1997;75:827–846. doi:10.1139/z97-106 [Google Scholar]
- Hurlbert A.H, Jetz W. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl Acad. Sci. USA. 2007;104:13 384–13 389. doi: 10.1073/pnas.0704469104. doi:10.1073/pnas.0704469104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson G.E. Homage to Santa Rosalia, or why are there so many kinds of animal? Am. Nat. 1959;93:145–159. doi:10.1086/282070 [Google Scholar]
- Jarvinen O. Species-to-genus ratios in biogeography: a historical note. J. Biogeogr. 1982;9:363–370. doi:10.2307/2844723 [Google Scholar]
- Kembel S.W, Hubbell S.P. The phylogenetic structure of a neotropical forest tree community. Ecology. 2006;87:S86–S99. doi: 10.1890/0012-9658(2006)87[86:tpsoan]2.0.co;2. doi:10.1890/0012-9658(2006)87[86:TPSOAN]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kraft N.J.B, Cornwell W.K, Webb C.O, Ackerly D.D. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat. 2007;170:271–283. doi: 10.1086/519400. doi:10.1086/519400 [DOI] [PubMed] [Google Scholar]
- Lovette I.J, Hochachka W.M. Simultaneous effects of phylogenetic niche conservatism and competition on avian community structure. Ecology. 2006;87:S14–S28. doi: 10.1890/0012-9658(2006)87[14:seopnc]2.0.co;2. doi:10.1890/0012-9658(2006)87[14:SEOPNC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Palmeirim J.J.M. Trophic structure of a neotropical frugivore community: is there competition between birds and bats? Oecologia. 1989;79:403–411. doi: 10.1007/BF00384321. doi:10.1007/BF00384321 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2007. R: a language and environment for statistical computing. [Google Scholar]
- Rodríguez J, Hortal J, Nieto M. An evaluation of the influence of environment and biogeography on community structure: the case of Holarctic mammals. J. Biogeogr. 2006;33:291–303. doi:10.1111/j.1365-2699.2005.01397.x [Google Scholar]
- Roth V.L. Cranial integration in the Sciuridae. Am. Zool. 1996;36:14–23. [Google Scholar]
- Schoener T.W. Field experiments on interspecific competition. Am. Nat. 1983;122:240–285. doi:10.1086/284133 [Google Scholar]
- Sechrest, W. 2003 Global diversity, endemism and conservation of mammals. PhD thesis, Department of Biology, University of Virginia, Charlottesville, Virginia.
- Swenson N.G, Enquist B.J, Pither J, Thompson J, Zimmerman J.K. The problem and promise of scale dependency in community phylogenetics. Ecology. 2006;87:2418–2424. doi: 10.1890/0012-9658(2006)87[2418:tpapos]2.0.co;2. doi:10.1890/0012-9658(2006)87[2418:TPAPOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Underwood A.J. What is a community? In: Raup D.M, Jablonski D, editors. Patterns and processes in the history of life. Springer; Berlin, Germany: 1986. pp. 351–367. [Google Scholar]
- Vos, R. A. 2006 Inferring large phylogenies: the big tree problem. PhD thesis, pp. 306, Department of Biological Sciences, Simon Fraser University.
- Webb C.O. Exploring the phylogenetic structure of ecological communities: an example for rainforest trees. Am. Nat. 2000;156:145–155. doi: 10.1086/303378. doi:10.1086/303378 [DOI] [PubMed] [Google Scholar]
- Webb C.O, Ackerly D.D, McPeek M.A, Donoghue M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 2002;33:475–505. doi:10.1146/annurev.ecolsys.33.010802.150448 [Google Scholar]
- Wilson D.E, Reeder D.A.M. Smithsonian Institution Press; Washington, DC: 1993. Mammal species of the world: a taxonomic and geographic reference. [Google Scholar]
- Wilson D.E, Reeder D.A.M. Smithsonian Institution Press; Washington, DC: 2005. Mammal species of the world: a taxonomic and geographic reference. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.