Abstract
Flowering plants have evolved multigene families of the class XI myosin motors, the functions of which remain poorly understood. Here, we investigated functional profiles of the Arabidopsis myosins that belong to two paralogous pairs, XI-K/XI-1 and XI-2/XI-B, using single and double gene-knockout mutants. It was found that the myosins XI-K, XI-2, and XI-B, but not XI-1 have overlapping and additive roles in the root hair elongation. A nonidentical set of the three myosins, XI-K, XI-1, and XI-2, exhibited partially redundant and additive roles in the transport of Golgi stacks, peroxisomes, and mitochondria. Conspicuously, the double xi-k/1 knockout plants that showed the largest cumulative reduction of the organelle velocities also exhibited a stunted plant growth and reduced fecundity phenotype. Collectively, these results suggest that the rapid, myosin-powered organelle trafficking is required for the optimal plant growth, whereas a distinct myosin function, presumably the vesicular transport, is involved in elongation of the root hairs. In addition, our data imply that the myosin gene duplication in plants has been followed by a gradual functional specialization of the resulting pairs of myosin paralogs.
Keywords: myosin, organelle transport, plant development
Plants exhibit the ultimate case of intracellular dynamics with the most rapid known trafficking of the membrane bound organelles such as mitochondria and peroxisomes (1, 2). Even the Golgi apparatus that is typically located in the nuclear vicinity of the animal and fungal cells is fragmented and highly mobile in the plant cells (3, 4). It has been known for a long time that the plant intracellular dynamics is powered primarily by the actomyosin motility system. Also for a long time, organelle trafficking in plants was interpreted as ‘cytoplasmic streaming,’ an indiscriminate cytosol flow that carries the organelles (5, 6). However, neither the physiological significance of incessant organelle motility nor the identities of myosin motors responsible for this phenomenon were known.
The sequencing of Arabidopsis and other plant genomes revealed that flowering plants possess myosins that belong to classes VIII and XI, with the latter class being the largest (7–9). Phylogenetic analysis showed that the class XI myosins form five conserved lineages, suggesting that the last common ancestor of the flowering plants had at least five myosins. Depending on the plant species, each of these lineages has from one to several closely related paralogous members that evolved via gene duplication (9). Whereas some of these duplications are evolutionarily recent resulting in almost identical myosins (e.g., in rice), others form more divergent groups of paralogs suggesting an earlier origin and functional specialization. Although indirect data such as subcellular localization or biophysical properties suggested involvement of class XI myosins in organelle motility (10), no specific functions were assigned to any plant myosin until very recently.
Perhaps, the most unexpected outcome of our study in which each of the 13 genes encoding class XI myosins in Arabidopsis was inactivated via insertional mutagenesis was that none of the resulting knockout lines exhibited obvious developmental abnormalities under optimal growth conditions (11). Given that myosins are essential for the viability of unicellular eukaryotes such as yeast (12), this result attested to the functional redundancy of plant myosins.
Interestingly, careful examination of the mutant plants revealed that inactivation of the myosins XI-K or XI-2 resulted in similar changes in cell physiology. First, the corresponding knockout mutants had much shorter root hairs (11). For the myosin XI-K, this result was independently obtained by another group (13). Second, it was found that the velocities of the Golgi stacks, peroxisomes, and mitochondria were reduced in root hairs and leaf cells of the xi-k and xi-2 knockout plants (11). The fact that root hairs are nonessential for plant growth (14) readily explains the lack of discernible developmental phenotype in these two knockouts. Furthermore, the root hair cells could be the most sensitive indicators of the myosin function because the rapid elongation of these cells heavily relies on the actomyosin-powered transport processes (15). It also seems intriguing that even the severalfold reduction in the velocities of three essential organelles did not cause significant developmental abnormalities (11).
An additional outcome of these studies was a dramatic reevaluation of the cytoplasmic streaming concept. Previous work on Golgi transport has already questioned the general applicability of this concept to higher plants (3). Our studies of the trafficking of Golgi, peroxisomes, and mitochondria in Nicotiana benthamiana (9) and Arabidopsis (11) revealed that each of these organelles exhibits multidirectional saltatory movements with a broad range of velocities, a pattern incompatible with the unidirectional cytoplasmic flow at a constant speed. Therefore, we proposed to replace the cytoplasmic streaming concept with the more mechanistically sound paradigm of cytoplasmic stirring caused by myosin-powered organelle hopping (9).
The results of our work are also incompatible with the simplistic notion of a unique function of each individual myosin in a particular transport process as a default explanation for the multiplicity of class XI myosins in plants. Instead, two myosins that belong to distinct evolutionary lineages, myosins XI-K and XI-2, were found to share functions in trafficking of at least two distinct organelles and in root hair elongation, whereas the remaining class XI myosins were assigned none of these functions [(9, 11) and unpublished data]. To address the significance of myosin proliferation in plants, here we further characterize the functions of two paralogous pairs of myosins, XI-K/XI-1 and XI-2/XI-B, using single and double gene-knockout mutants. We show that these paralogs exhibit partially redundant but nonidentical functional profiles. Furthermore, we provide an experimental evidence for the physiological relevance of the organelle trafficking in plants.
Results
Elimination of the Myosins XI-K and XI-1 Results in Defective Plant Growth.
Evolutionary conservation of myosins throughout the plant kingdom, from algae to mosses to flowering plants attests to their essential role in plant physiology (8, 9). On the other hand, lack of discernible developmental phenotypes in the single myosin gene knockouts suggested that the functions of individual myosins were partially redundant (11). Therefore, it could be expected that inactivation of more than one myosin gene is required to unveil the roles of myosin motors in plant development. Here, we characterized Arabidopsis double myosin gene-knockout mutants that affected two pairs of closely related paralogs, myosins XI-K/XI-1 and XI-2/XI-B. Three of these myosins, XI-K, XI-1, and XI-2, are among the four most abundant myosins in Arabidopsis leaves (http://www.weigelworld.org/resources).
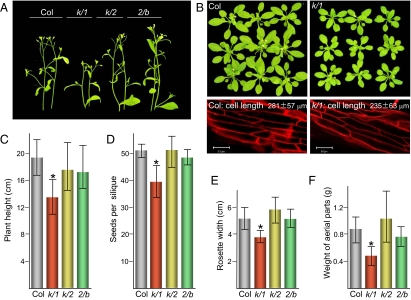
The double myosin gene knockout lines xi-k/1, xi-k/2, and xi-2/b were generated by crossing the corresponding single knockouts that were characterized earlier (11), self-pollinating the resulting heterozygous plants, and PCR screening for homozygous progeny. Examination of the overall morphology of the mutant plants grown under optimal conditions revealed a readily discernible stunted phenotype in the xi-k/1 double knockout, but not the other mutants (Fig. 1 A and B). The quantification of the plant growth parameters demonstrated that the mean plant height and width of the leaf rosette of the xi-k/1 mutant were reduced to approximately 70% of those in the wild type (Fig. 1 A–C and E). Correspondingly, the mean weight of the plant aerial parts of this double knockout was <60% of that in the wild type (Fig. 1F). It should be emphasized that the differences in these parameters of the plant growth between the xi-k/1 mutant and the wild type plants or the other two mutant plant lines were highly statistically significant (P < 0.001). Furthermore, the multiple comparison tests revealed that the xi-k/1 line was the only one whose datasets formed a distinct Scheffé group in the analyses of plant height, rosette width, and plant weight (supporting information (SI) Table S1). No obvious morphological abnormalities were detected in any of the double knockout mutants (Fig. 1 A and B).
Fig. 1.
Growth phenotypes of the Arabidopsis double knockout plants in which two class XI myosin genes were inactivated. (A) Pairs of the inflorescence shoots of the plants sown on the same day (phase 6.0–6.1). (B) Top row, leaf rosettes of the groups of nine plants sown on the same day (phase 5–6). Bottom row, representative images of the leaf epidermal cell files used for measurements of the mean cell length shown within panels. (Scale bars, 50 μm.) (C) Mean plant height. (D) Number of seeds per silique. (E) Leaf rosette width. (F) Weight of the aerial parts. Col, the parental Columbia-0 line; k/1, k/2, and 2/b, double knockouts xi-k/1, xi-k/2, and xi-2/b, respectively. Asterisks mark the columns representing data that are significantly different (P < 0.001) from all others in a corresponding set.
To determine whether the average cell size is also affected in the xi-k/1 plants, we measured the elongated leaf cells present at the adaxial midrib epithelium. It was found that this size was ≈84% (P < 0.001) that of the parental Columbia-0 plants (Fig. 1B, bottom panels). Because the overall leaf rosette width in this variant was reduced to ≈70%, we conclude that both the smaller cell size and fewer cell numbers contribute to the reduced stature of the xi-k/1 plants.
The quantification of the mean number of the seeds per silique also showed an approximate 20% reduction relative to the wild type for xi-k/1 but not for the other mutants (Fig. 1D). Statistical analysis confirmed that the xi-k/1 line formed a separate Scheffé group whereas the Columbia-0 and two other mutant lines fell into the same group (Table S1). These results demonstrated that the elimination of a pair of myosin paralogs XI-K and XI-1 affected not only the overall growth performance but also fecundity of the mutant plants. The following experiments were designed to gain a mechanistic insight into the myosin-powered transport processes that are required for the optimal plant productivity.
Overlapping Roles of Myosins XI-K, XI-1, and XI-2 in Organelle Motility.
Our previous work demonstrated that the inactivation of the Arabidopsis myosins XI-K or XI-2 resulted in an approximate 3- to 4-fold reduction in the velocities of Golgi stacks and peroxisomes in the leaf cells. Studies of the mitochondrial transport revealed a similar 4-fold velocity reduction in the myosin xi-k knockout plants, while only a modest, an approximate 10% reduction was observed in the myosin xi-2 knockout plants (11). Interestingly, limited study of the most closely related paralogs of the myosins XI-K and XI-2, myosins XI-1 and XI-B, respectively, did not reveal any significant reduction in the mean organelle velocities (11). These results suggested that the myosins XI-K and XI-2 share the functions of Golgi and peroxisomal transport, although the former myosin is a principal motor that moves mitochondria. However, the potential involvement of myosins XI-1 and XI-B into organelle motility could not be rigorously excluded with the available data.
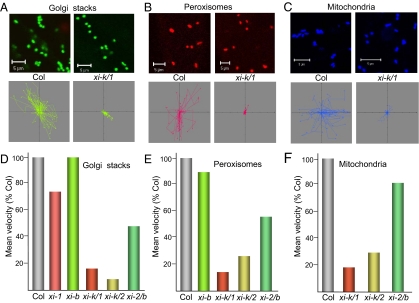
To determine whether there is a correlation between organelle trafficking and plant growth phenotype described above, we systematically measured mean velocities of Golgi, peroxisomes, and mitochondria in the additional single and double knockout mutants (Fig. 2). These experiments also clarified the relative contributions of the myosins XI-K, XI-1, XI-2, and XI-B into organelle trafficking. The myosin gene knockout lines were transformed with the Golgi-specific reporter fused to the yellow fluorescent protein (Fig. 2A) or the peroxisome-specific reporter fused to the red fluorescent protein mCherry (Fig. 2B). The mitochondria were visualized by using a vital dye (Fig. 2C). More than two hundred of individual organelles for each experimental variant were traced by using time-lapse laser scanning confocal microscopy (e.g., Fig. 2 A–C, bottom row), and their velocities were measured by using Volocity software.
Fig. 2.
Motility of the Golgi stacks (A and D), peroxisomes (B and E), and mitochondria (C and F) in the leaf epidermal cells of the single and double myosin gene knockout plants. (A–C, upper row), representative 2-D organelle images obtained by using confocal laser scanning microscope and used to trace individual organelles (corresponding tracks are shown in bottom row) and measure their velocities. (A–C, bottom row), movement of organelles present in the above images plotted to a common origin. Note much shorter tracks because of reduced organelle velocities in the xi-k/1 mutant compared with those in Columbia-0. (D–F) Mean velocities of the organelles expressed as percentage of those in the parental Columbia-0 (Col) line. The color code is the same as in Fig. 1. (Scale bars, 5 μm.)
Analysis of the velocities of the Golgi stacks in the single knockouts xi-1 and xi-b showed that the myosin XI-1, but not XI-B provides a moderate contribution into the motility of this organelle (Fig. 2D). The fact that the Golgi velocity in a double xi-2/b knockout (Fig. 2D) was not significantly different from that in a single xi-2 knockout further supported this conclusion (11). We also found that the myosins XI-K and XI-2 have additive functions in the motility of Golgi stacks. Indeed, the mean Golgi velocity in the xi-k/2 mutant was only ≈9% of that in the wild type (Fig. 2D) compared with ≈35% in the corresponding single knockouts (11). The xi-k/1 double knockout also showed reduced Golgi velocity compared with that of the single knockouts (17%, 34%, and 73% of that in the wild type for the xi-k/1, xi-k, and xi-1 knockouts, respectively) (Fig. 2 A and D) (11). All differences between mean velocities of Golgi in double knockouts and those in the wild type or single knockouts were highly statistically significant (P < 0.001). The multiple comparison tests also confirmed this conclusion (Table S2). These results indicated that although the myosins XI-K and XI-2 provide the largest independent contributions into Golgi transport, myosin XI-1 could also contribute to this process, at least in the absence of its closest paralog, myosin XI-K.
The velocities of peroxisomes in the knockout lines xi-b and xi-2/b were 88% and 55% of that in the wild-type plants (Fig. 2E). Unexpectedly, the velocity of peroxisomes in the double xi-k/2 knockout (23% of that in wild type; Fig. 2E) was virtually identical to that in a single xi-k knockout (11) indicating that, in the absence of myosin XI-K, myosin XI-2 does not contribute substantially to the peroxisome trafficking. In contrast, inactivation of the myosin XI-K and its paralog XI-1 in the xi-k/1 double knockout resulted in a significant reduction of the peroxisome mean velocity: 14% of that in the wild type (Fig. 2 B and E) compared with 23% and 80% for the single xi-k and xi-1 knockouts, respectively (11). Statistical analysis confirmed highly significant differences between the two double knockout mutants (Table S2). These results showed that the relative contributions of the myosins XI-K, XI-1 and XI-2 into peroxisome transport are additive for the myosin paralogs XI-K and XI-1, but not for the myosin XI-2, and that myosin XI-B does not play a significant role in peroxisome transport.
Investigation of the mitochondrial motility in double knockouts revealed that, in the absence of myosin XI-K, inactivation of the myosin XI-1 but not XI-2 did further reduce the mean velocity of this organelle (Fig. 2 C and F; see Table S2 for the statistical analysis). In the case of the double knockout xi-2/b, the mean mitochondrial velocity was not significantly different from those in corresponding single mutants: 81% (Fig. 2F) versus 89% and 85%, respectively (11). These results imply that the mitochondrial motility depends chiefly on myosin XI-K with a moderate contribution of the paralogous myosin XI-1 and only marginal if any contributions of another paralogous pair, myosins XI-2 and XI-B.
Our examination of organelle movement in myosin gene knockout lines revealed a range of myosin contribution patterns from additive in the case of Golgi to a complex and seemingly interdependent in the case of peroxisomes. The most important outcome of these experiments was a correlation between the organelle motility and the plant growth. Indeed, the xi-k/1 mutant that showed stunted growth also exhibited the largest cumulative reduction in the velocities of all three types of organelles, Golgi, peroxisomes, and mitochondria, among the three double knockouts (Fig. 2 A–F). In contrast, xi-k/2 mutant in which the velocity of Golgi stacks but not peroxisomes or mitochondria was reduced compared with those in the single knockout mutants (Fig. 2 D–F), did not possess a stunted growth phenotype (Fig. 1 A and C–F). These data strongly suggest a causal link between the rapid trafficking of at least three distinct organelles and plant development.
Myosins XI-K, XI-2, and XI-B Contribute to Elongation of the Root Hairs.
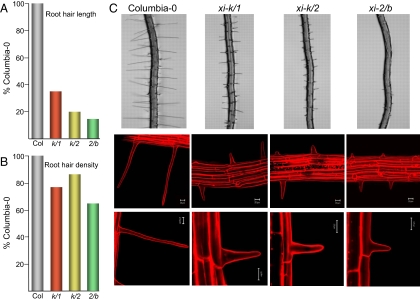
Previous examination of the Arabidopsis single myosin gene knockout mutants revealed that the myosins XI-K and XI-2 but not the remaining 11 class XI myosins including XI-1 and XI-B are required for the growth of the root hairs (11). Here, we analyzed the root hair length in the double knockout mutants xi-k/2, xi-k/1, and xi-2/b. It was found that simultaneous elimination of the myosins XI-K and XI-2 resulted in root hairs whose mean length was only 20% of the wild type (Fig. 3 A and C) compared with ≈30–40% in the single xi-k and xi-2 mutants (11), suggesting the additive roles of these myosins in the root hair elongation. In contrast, the mean root hair length in the double xi-k/1 knockout, 35% of the wild type (Fig. 3 A and C), was similar to that for a single xi-k knockout (11) indicating that the myosin XI-1 does not provide a significant contribution to the root hair growth. Surprisingly, even though elimination of the myosin XI-B alone had no effect on root hairs (11), the double knockout xi-2/b had the shortest root hairs among all mutants characterized so far at 15% of the wild type value (Fig. 3 A and C).
Fig. 3.
Root hair phenotypes. Mean root hair length (A) and density (B) in the double knockout lines expressed as percentage of those in the control (Col, the parental Columbia-0 line). Designations and color code are the same as in Fig. 1. Root hair morphology (C) in the parental Columbia-0 line and the double knockout mutant lines. Top row, images taken under visible light by using stereoscope. Middle and bottom rows, images taken under UV by using confocal laser scanning microscope. (Scale bars, 20 μm.)
Further characterization of the double knockout mutants revealed that neither overall morphology nor basal position of the root hairs relative to hair-forming cells (trichoblasts) was different from those in the wild-type root hairs (Fig. 3C). Furthermore, the density of the root hairs was also similar in the mutant and wild type plants (Fig. 3B). These results suggested that the initiation of hair buds on the trichoblasts or the following transition to the tip growth were unaffected in these mutants. It can be concluded that the reduction in the root hair length in the myosin knockouts was due primarily to the defective cell elongation rather than establishment of cell polarity. In addition, these data showed that the myosins XI-K, XI-2, and XI-B, but not XI-1, provide additive contributions to the rapid growth of root hairs.
It should be emphasized that there was no correlation between the root hair length and overall plant growth. Although the xi-2/b mutant plants had the shortest root hairs among the mutants (Fig. 3A), they exhibited no reduction in biomass (Fig. 1F). Conversely, the xi-k/1 plants that had the longest root hairs among the double knockouts did exhibit reduced growth and fecundity.
Discussion
Myosins are ubiquitous molecular motors that diversified into several evolutionary lineages with distinct functions and representation among eukaryotic organisms (16). One of the major myosin lineages includes class V present in fungi and animals and class XI present in plants. In addition to the N-terminal motor domains conserved in all myosins, class V and XI myosins share the ‘Diluted’ (DIL) motif that is present in the C-terminal cargo-binding domain (9, 17). These structural similarities suggest conservation of at least some functions between class V and XI myosins (18).
Most of the information on class V myosins was obtained in the yeast where myosin Myo2p was found to be required for partitioning peroxisomes, vacuoles, and possibly mitochondria among the dividing cells (12, 19). It is not known, however, whether these organelles are motile in the nondividing yeast cells. In contrast, plant peroxisomes, mitochondria, and Golgi stacks exhibit extremely rapid and incessant, actomyosin-dependent, trafficking. Our recent work has shown that this trafficking relies on at least two class XI myosins, XI-K and XI-2 in two plant species, N. benthamiana (9) and Arabidopsis (11). Here, we show that the third myosin, XI-1, that is the closest paralog of XI-K, also contributes to motility of Golgi, peroxisomes, and mitochondria.
Interestingly, the relative contributions of the myosins XI-K, XI-1, and XI-2 into organelle motility are complex and sometimes interdependent. The simplest case is the Golgi trafficking to which all three myosins provide apparently additive contributions. In the case of peroxisomes, myosins XI-K and XI-1 appear to cooperate, whereas the involvement of myosin XI-2 is apparent only if myosin XI-K is present. This seemingly paradoxical result suggests that efficient binding of peroxisomes by myosin XI-2 requires myosin XI-K, for example, via the formation of a heterodimer. By and large, trafficking of mitochondria relies on myosin XI-K with myosin XI-1 providing a moderate contribution, and myosins XI-2 and XI-B, perhaps, playing marginal roles.
Although the rapid trafficking of plant organelles is among the most prominent features of plant cell biology that has been known for over one-half century (5), the functional significance of this process has not been addressed experimentally. The analysis of myosin gene knockouts presented here suggested that the high-velocity transport of organelles is required for the optimal plant growth. This hypothesis is supported by the correlation between cumulative negative effect on the trafficking of Golgi stacks, peroxisomes, and mitochondria and reduced plant growth and fecundity in the myosin xi-k/1 double gene knockout. An alternative explanation, namely, involvement of myosins XI-K and XI-1 in a distinct transport processes such as endoplasmic reticulum dynamics or vesicular trafficking (4) is also compatible with the existing data. The fact that despite the substantial reduction of organelle velocities in the xi-k, xi-2, and xi-k/2 plants, no developmental phenotypes were observed for these mutants suggests that these velocities need to go beyond a threshold level to measurably affect plant growth.
It is important to emphasize that no correlation was found between the elongation of the root hairs on one hand, and the organelle motility or the overall plant growth on the other. First, the fact that more than sixfold reduction in the mean root hair length seen in the xi-2/b double knockout did not affect plant growth is well compatible with the known dispensability of root hairs for plant development (14). Second, myosin XI-1 has been implicated in the motility of all three organelles but not the root hair elongation, whereas the opposite is true for the myosin XI-B. Perhaps the simplest mechanistic interpretation of the specific involvement of myosin XI-B into root hair elongation but not the organelle trafficking is that this myosin contributes to transport of the secretory vesicles that deliver materials for the root hair tip growth (20, 21).
Our results provide an experimental insight into evolution of the multigene myosin family in flowering plants via characterization of the functional profiles of two paralogous myosin pairs. Interestingly, even though these pairs belong to distinct evolutionary lineages within class XI, there is a substantial functional overlap between them as indicated by the similar roles of myosins XI-K and XI-2 in Golgi transport and root hair elongation. At the same time, contributions of these myosins in the mitochondrial transport are very different in magnitude. Comparison of the myosin functions within the paralogous pairs also shows substantial differences: Myosin XI-K but not XI-1 plays a role in root hair elongation, whereas the myosin XI-2 but not XI-B is involved in organelle trafficking. Mechanistically, these differential roles of myosins could be explained by the changes in their cargo-binding specificities and tissue-specific regulation of the expression levels. Taken together, the described functional redundancies of the class XI myosins and a degree of specialization among the most closely related paralogs indicate that diversification of the class XI myosins in flowering plants is an ongoing evolutionary process.
Materials and Methods
T-DNA Insertion Mutants.
The Arabidopsis thaliana ecotype Columbia-0 homozygous single knockout lines with the inactivated genes encoding myosins XI-K (SALK 067972; At5g20490), XI-1 (SALK 019031; At1g17580), XI-2 (SALK 055785; At5g43900), and XI-B (SALK 113062; At1g04160) were described earlier (11). To generate the double knockout mutants xi-k/1, xi-k/2, and xi-2/b, the corresponding homozygous lines were crossed. The F1 progeny was allowed to self-pollinate, and the F2 progeny was screened by PCR for the presence of both mutant alleles and for the absence of both wild-type alleles. The offspring of the double-homozygous plants was again screened by PCR to demonstrate lack of segregation for each of the mutant loci. In addition, the RT-PCR as described in ref. 11 was used to confirm that the wild-type mRNA sequence for each of the inactivated myosins is disrupted in the double-homozygous mutants.
Plant Growth Phenotypes.
Seeds collected from either wild-type Columbia-0 or double-homozygous plants carrying the T-DNA insertions in two myosin genes simultaneously were cold stratified for 3 days and planted in 72-cell flats. After germination the seedlings were thinned to retain one plant per well and grown in a greenhouse under standard conditions for six weeks (growth stages 6.3–6.5) (22). Plants were harvested and the following measurements were performed for each plant: total weight of aerial parts, plant height, and diameter of the leaf rosette (major axis). In addition, the fully developed siliques were collected and soaked overnight in 95% ethanol to remove chlorophyll. The numbers of seeds in each silique were determined by using a dissecting stereomicroscope. A sample size for each plant line analyzed was approximately 60 plants.
Statistical Analyses.
To determine the statistical significance of the observed differences in the plant height and weight, rosette width, and the numbers of seeds per silique, a general linear model analysis followed by Scheffé's multiple comparison test were performed. This approach was used because of the variable numbers of replicates between the treatments. The logarithmic transformation was performed before the analysis to achieve homoscedascity of the variances for each set of data. This transformation also reduced the positive skewness of the data and therefore approached normality. In addition to the mean values, the medians were also determined to obtain a central measurement that is not significantly affected by the skewness (Table S1).
Arabidopsis Transformation.
The homozygous plants of the single knockout lines xi-1 and xi-b and the double knockout lines xi-k/1, xi-2/b, and xi-k/2 described above were transformed to express the Golgi-specific reporter that represented a fusion of the rat A-2,6-sialyltransferase to the yellow fluorescent protein (11, 23). Analogously, the peroxisome-specific fusion reporter between mCherry and a signal peptide of the pumpkin hydroxypyruvate reductase has been used to transform xi-b, xi-k/1, xi-2/b, and xi-k/2 mutant lines (11, 24). The resulting T1 plant lines exhibiting Hygromycin resistance were grown, and the transgenic lines expressing organelle markers were selected by using epifluorescent microscopy.
Organelle Trafficking.
The time-lapse confocal laser scanning microscopy was done using a Zeiss 510 Meta (Zeiss) microscope as described in ref. 9. The consecutive 2D images were taken at 2-sec intervals for Golgi and peroxisomes or 1 sec for mitochondria. The fluorescent, reporter-labeled, Golgi stacks and peroxisomes were observed in the leaf epidermal cells using the following configurations of excitation and emission filters respectively: 513 nm and 527 nm for the yellow fluorescent protein and 587 nm and 610 nm for mCherry. Live mitochondria were visualized by using 50 nM Rhodamine 123 (Invitrogen). Tracking and measurements of velocities of individual organelles was performed by using the Volocity 3.7.0 Classification software (Improvision, Image Processing and Vision Company, Ltd.) that captures all fluorescent organelles present in each image and tracks them based on their distinct shape and brightness (Fig. 2 A–C). Organelles that leave the focal plane during image acquisition are not followed any further resulting in a potential underestimation of the mean velocity. In addition, the organelle tracks acquired by software were edited manually to exclude infrequent erroneous tracks that originated because of misidentification. At least 250 individual organelles were analyzed for each experimental variant. The statistical analysis of the organelle velocities was done by using a general linear model analysis followed by Scheffé's multiple comparison tests as detailed above (Table S2).
Root Hair Length, Density, and Morphology.
The seeds were grown on vertical plates containing 0.5 × Murashige and Skoog medium, 5 mM Mes (pH 5.8), 1% sucrose, and 0.6% Phytogel under a 16-h light/8-h dark cycle. Root hairs of the 5-day-old seedlings were photographed by using Leica MZ6 stereozoom microscope equipped with a CCD camera and measured by using the Image-Pro (Media Cybernetics, Inc.) software. At least two hundred root hairs from four different plants were measured to determine the mean lengths and densities for each plant line shown in Fig. 3. To image the root hair cell walls, the 4-day-old roots were incubated in 10 μg/ml propidium iodide (MP Biomedical) for 10 min and subjected to confocal laser scanning microscopy using 530-nm excitation and 620-nm filters.
Supplementary Material
Acknowledgments.
We thank Dr. Eugene V. Koonin for critical reading of the manuscript, Dr. Dror Avisar for his assistance with the statistical analyses, and Drs. Galina Bulatova and Vladimir Bulatov for their help with photography. We acknowledge the Confocal Microscopy Facility of the OSU Center for Genome Research and Biocomputing. The publication of this article was made possible in part by National Institutes of Health Grant 1S10RR107903–01.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810730105/DCSupplemental.
References
- 1.Wada M, Suetsugu N. Plant organelle positioning. Curr Opin Plant Biol. 2004;7:626–631. doi: 10.1016/j.pbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Shimmen T, Yokota E. Cytoplasmic streaming in plants. Curr Opin Plant Biol. 2004;16:68–72. doi: 10.1016/j.ceb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Nebenfuhr A, Staehelin LA. Mobile factories: Golgi dynamics in plant cells. Trends Plants Sci. 2001;6:160–167. doi: 10.1016/s1360-1385(01)01891-x. [DOI] [PubMed] [Google Scholar]
- 4.Matheson LA, Hanton SL, Brandizzi F. Traffick between the plant endoplasmic reticulum and Golgi apparatus: To the Golgi and beyond. Curr Opin Plant Biol. 2006;9:601–609. doi: 10.1016/j.pbi.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Shimmen T. The sliding theory of cytoplasmic streaming: Fifty years of progress. J Plant Res. 2007;120:31–43. doi: 10.1007/s10265-006-0061-0. [DOI] [PubMed] [Google Scholar]
- 6.Taiz L, Ziegler E. Plant Physiol. Sunderland, MA: Sinauer Assoc., Inc.; 2006. [Google Scholar]
- 7.Reddy AS, Day IS. Analysis of the myosins encoded in the recently completed Arabidopsis thaliana genome sequence. Genome Biol. 2001;2:0024. doi: 10.1186/gb-2001-2-7-research0024. RESEARCH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezanilla M, Horton AC, Sevener HC, Quatrano RS. Phylogenetic analysis of new plant myosin sequences. J Mol Evol. 2003;57:229–239. doi: 10.1007/s00239-003-2469-7. [DOI] [PubMed] [Google Scholar]
- 9.Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. Myosin XI-K is required for rapid trafficking of Glgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol. 2008;146:1098–1108. doi: 10.1104/pp.107.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y-RJ, Liu B. Cytoskeletal motors in Arabidopsis. Sixty-one kinesins and seventeen myosins. Plant Physiol. 2004;136:3877–3883. doi: 10.1104/pp.104.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peremyslov VV, Prokhnevsky AI, Avisar D, Dolja VV. Two class XI myosins function in organelle trafficking and root hair development in Arabidopsis. Plant Physiol. 2008;146:1109–1116. doi: 10.1104/pp.107.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretcher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- 13.Ojangu EL, Jarve K, Paves H, Truve E. Arabidopsis thaliana myosin XIK is involved in root hair as well as trichome morphogenesis on stems and leaves. Protoplasma. 2007;230:193–202. doi: 10.1007/s00709-006-0233-8. [DOI] [PubMed] [Google Scholar]
- 14.Carol RJ, Dolan L. Building a hair: Tip growth in Arabidopsis thaliana root hairs. Philos Trans R Soc Lond B Biol Sci. 2002;357:815–821. doi: 10.1098/rstb.2002.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith LG, Oppenheimer DG. Spatial control of cell expansion by the plant cytoskeleton. Annu Rev Cell Dev Biol. 2005;21:271–295. doi: 10.1146/annurev.cellbio.21.122303.114901. [DOI] [PubMed] [Google Scholar]
- 16.Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- 17.Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci USA. 2006;103:3681–3686. doi: 10.1073/pnas.0506307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JF, Nebenfuhr A. Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites. J Biol Chem. 2007;282:20593–20602. doi: 10.1074/jbc.M700645200. [DOI] [PubMed] [Google Scholar]
- 19.Valiathan RR, Weisman LS. Pushing for answers: Is myosin V directly involved in moving mitochondria. J Cell Biol. 2008;181:15–18. doi: 10.1083/jcb.200803064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annu Rev Cell Dev Biol. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- 21.Cole RA, Fowler JE. Polarized growth: Maintaining focus on the tip. Curr Opin Plant Biol. 2006;9:579–588. doi: 10.1016/j.pbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Boyes DC, et al. Growth stage–based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saint-Jore CM, Evins J, Batoko H, Brandizzi F, Moore I, Hawes C. Redistribution of membrane proteins between the Golgi apparatus and endoplasmic reticulum in plants is reversible and not dependent on cytoskeletal networks. Plant J. 2002;29:661–678. doi: 10.1046/j.0960-7412.2002.01252.x. [DOI] [PubMed] [Google Scholar]
- 24.Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M. Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: Dynamic morphology and actin-dependent movement. Plant Cell Physiol. 2002;43:331–341. doi: 10.1093/pcp/pcf037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.