Abstract
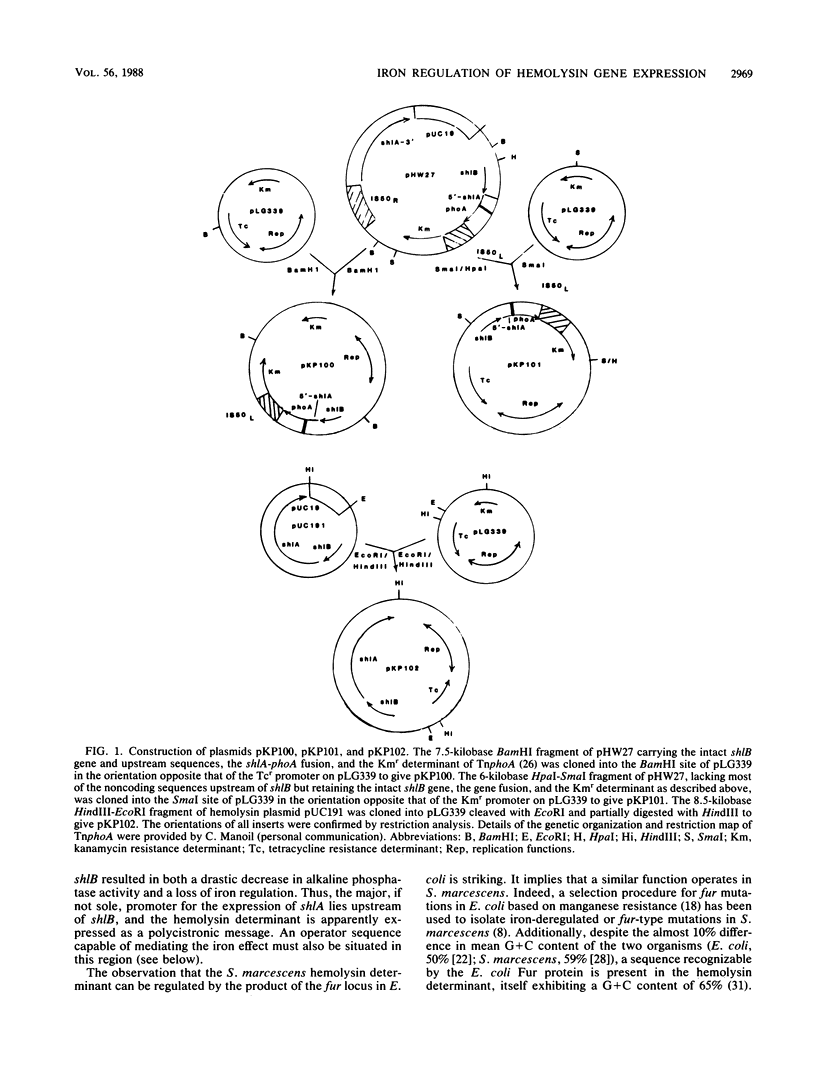
The hemolytic activity of Serratia marcescens was examined as a function of iron availability. Restriction of iron by the nonmetabolizable chelator 2,2'-dipyridyl or the iron-binding protein transferrin produced a marked increase in hemolytic activity. The hemolytic activity of S. marcescens is determined by two adjacent genes, 5'-shlB-shlA-3', where shlA encodes the hemolysin which requires the ShlB protein for activity. A gene fusion between the promoter-proximal portion of shlA and phoA, the Escherichia coli alkaline phosphatase gene, was subcloned into a medium-copy-number vector, and the recombinant plasmid was introduced into S. marcescens. The expression of shlA was measured as a function of alkaline phosphatase activity, which increased threefold under iron-restricted conditions. Removal of the 5' noncoding region upstream of shlB in the fusion vector resulted in a 10-fold decrease in alkaline phosphatase activity under iron-sufficient conditions, with no effect of iron limitation on this residual activity. This suggested that the site mediating iron regulation of shlA expression occurs upstream of shlB. Consistent with this, we observed iron-regulated synthesis of the ShlB protein in Western immunoblots of isolated outer membranes. The hemolysin determinant was subsequently expressed on a medium-copy-number vector in fur+/fur isogenic strains of E. coli K-12, where a 10-fold-higher activity was observed in the mutant strain compared with the wild type. A sequence exhibiting some homology to the Fur-binding consensus sequence was identified upstream of the shlB coding region, overlapping the -35 region of a putative promoter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan B. J., Stevenson R. M. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can J Microbiol. 1981 Oct;27(10):1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- Ball T. K., Saurugger P. N., Benedik M. J. The extracellular nuclease gene of Serratia marcescens and its secretion from Escherichia coli. Gene. 1987;57(2-3):183–192. doi: 10.1016/0378-1119(87)90121-1. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Braun V., Burkhardt R. Regulation of the ColV plasmid-determined iron (III)-aerobactin transport system in Escherichia coli. J Bacteriol. 1982 Oct;152(1):223–231. doi: 10.1128/jb.152.1.223-231.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Günther H., Neuss B., Tautz C. Hemolytic activity of Serratia marcescens. Arch Microbiol. 1985 May;141(4):371–376. doi: 10.1007/BF00428852. [DOI] [PubMed] [Google Scholar]
- Braun V., Neuss B., Ruan Y., Schiebel E., Schöffler H., Jander G. Identification of the Serratia marcescens hemolysin determinant by cloning into Escherichia coli. J Bacteriol. 1987 May;169(5):2113–2120. doi: 10.1128/jb.169.5.2113-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eaves G. N., Jeffries C. D. ISOLATION AND PROPERTIES OF AN EXOCELLULAR NUCLEASE OF SERRATIA MARCESCENS. J Bacteriol. 1963 Feb;85(2):273–278. doi: 10.1128/jb.85.2.273-278.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R. T., Jr, Booth J. W., Becker R. R. Uptake of iron from hemoglobin and the haptoglobin-hemoglobin complex by hemolytic bacteria. Int J Biochem. 1985;17(7):767–773. doi: 10.1016/0020-711x(85)90262-9. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Helms S. D., Oliver J. D., Travis J. C. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984 Aug;45(2):345–349. doi: 10.1128/iai.45.2.345-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Membrane glycoprotein receptor and hole-forming properties of a cytolytic protein toxin. Biochemistry. 1982 Mar 30;21(7):1662–1667. doi: 10.1021/bi00536a029. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., Faltin Y., Scheffer J., Schöffler H., Braun V. Role of cell-bound hemolysin as a pathogenicity factor for Serratia infections. Infect Immun. 1987 Nov;55(11):2554–2561. doi: 10.1128/iai.55.11.2554-2561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebek G., Gruenig H. M. Relation between the hemolytic property and iron metabolism in Escherichia coli. Infect Immun. 1985 Dec;50(3):682–686. doi: 10.1128/iai.50.3.682-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the Serratia marcescens lipoprotein gene. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1369–1373. doi: 10.1073/pnas.77.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrisius J., Bhakdi S., Roth M., Tranum-Jensen J., Goebel W., Seeliger H. P. Production of listeriolysin by beta-hemolytic strains of Listeria monocytogenes. Infect Immun. 1986 Jan;51(1):314–319. doi: 10.1128/iai.51.1.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Schiebel E., Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988 Jul;170(7):3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J. D., Stoufer S. D., Ogrydziak D. M. Efficient transformation of Serratia marcescens with pBR322 plasmid DNA. Gene. 1982 Jan;17(1):107–112. doi: 10.1016/0378-1119(82)90106-8. [DOI] [PubMed] [Google Scholar]
- Rennie R. P., Freer J. H., Arbuthnott J. P. The kinetics of erythrocyte lysis by Escherichia coli haemolysin. J Med Microbiol. 1974 May;7(2):189–195. doi: 10.1099/00222615-7-2-189. [DOI] [PubMed] [Google Scholar]
- Schmitz G., Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985 Mar;161(3):1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Hayden C. Secretion of phospholipase C by Pseudomonas aeruginosa. Infect Immun. 1979 Aug;25(2):558–564. doi: 10.1128/iai.25.2.558-564.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Timmis K., Winkler U. Isolation of covalently closed circular deoxyribonucleic acid from bacteria which produce exocellular nuclease. J Bacteriol. 1973 Jan;113(1):508–509. doi: 10.1128/jb.113.1.508-509.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Satoh T., Katsu T., Shinoda S. Mechanism of haemolysis by Vibrio vulnificus haemolysin. J Gen Microbiol. 1987 Oct;133(10):2859–2864. doi: 10.1099/00221287-133-10-2859. [DOI] [PubMed] [Google Scholar]



