Abstract
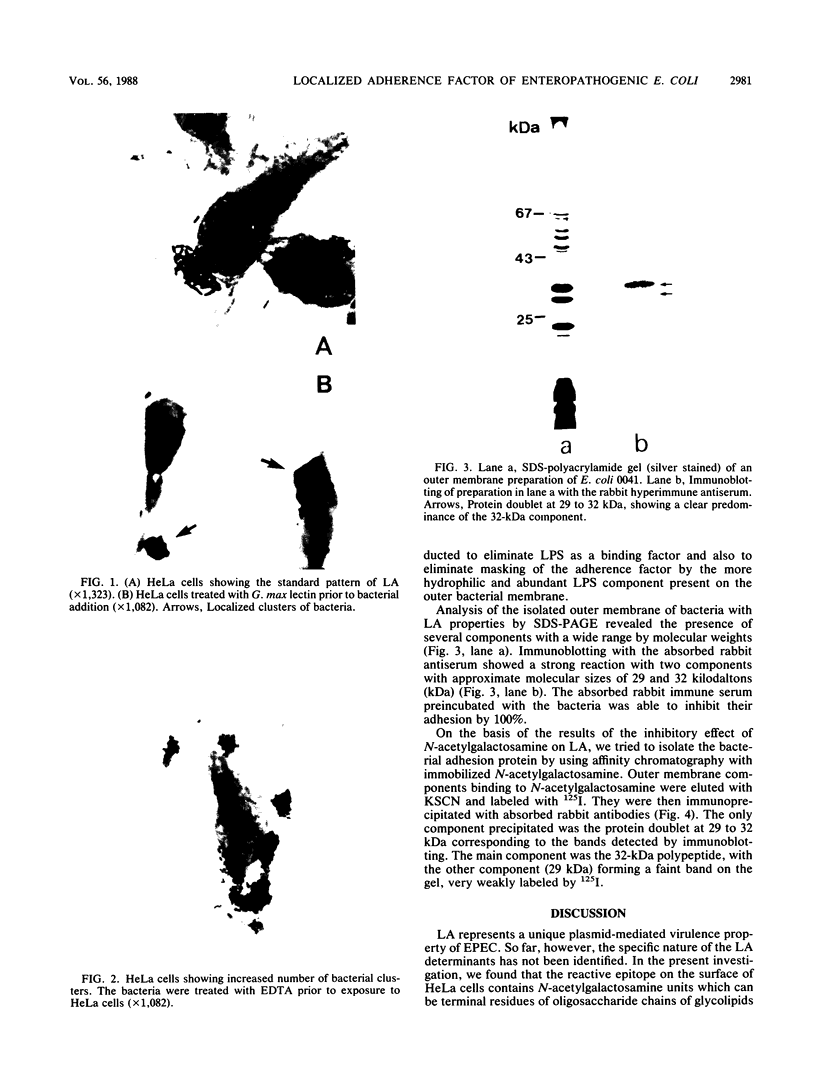
The binding factor of enteropathogenic Escherichia coli O111:H- responsible for localized adherence (LA) on HeLa cells was investigated. Inhibition of LA by carbohydrates and lectins showed that the reactive epitope on HeLa cells contains N-acetylgalactosamine units. Treatment of bacteria with EDTA for extraction of lipopolysaccharides eliminated these polymers as binding factors. Such treatment also caused a marked increase in adhesion suggesting steric hindrance by lipopolysaccharides of the LA factor binding capacity. Immunoblotting with rabbit antibodies showed a strong reaction with two components with approximate molecular sizes of 29 and 32 kilodaltons (kDa) present in the outer membrane preparations of bacteria. Both the absorbed rabbit immune serum and the outer membrane preparation of the bacteria inhibited bacterial adhesion by 100%. Outer membrane components were isolated from an N-acetylgalactosamine-agarose column by elution with KSCN, labeled with 125I, and immunoprecipitated with absorbed rabbit hyperimmune antiserum. The only component precipitated was the protein doublet at 29 to 32 kDa corresponding to the components detected by immunoblotting. The predominant component was always the 32-kDa polypeptide. We conclude that this component of the outer membrane is the best candidate for the LA factor in enteropathogenic E. coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Willshaw G. A., Rowe B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to enteropathogenic serogroups. J Gen Microbiol. 1988 May;134(5):1315–1321. doi: 10.1099/00221287-134-5-1315. [DOI] [PubMed] [Google Scholar]
- Clausen C. R., Christie D. L. Chronic diarrhea in infants caused by adherent enteropathogenic Escherichia coli. J Pediatr. 1982 Mar;100(3):358–361. doi: 10.1016/s0022-3476(82)80429-0. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Pácsa A. S., Emödy L., Vörös S., Sélley E. Modified enzyme-linked immunosorbent assay for detecting enteroinvasive Escherichia coli and virulent Shigella strains. J Clin Microbiol. 1985 Mar;21(3):415–418. doi: 10.1128/jcm.21.3.415-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Toledo M. R., Davis B. R., Blake P. A., Trabulsi L. R. Correlation between adherence to HeLa cells and serogroups, serotypes, and bioserotypes of Escherichia coli. Infect Immun. 1985 Sep;49(3):528–532. doi: 10.1128/iai.49.3.528-532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRABULSI L. R., MANISSADJAN A., PENNA H. A., LIBERATORE R., DUAILIBE L., de CAMARGO, PEIXOTO E. S. [Infantile diarrhea caused by enteropathogenic colibacilli. Preliminary studies on the occurrence of certain groups and serological types in Sao Paulo]. Rev Inst Med Trop Sao Paulo. 1961 Nov-Dec;3:267–270. [PubMed] [Google Scholar]
- Toledo M. R., Alvariza M. do C., Murahovschi J., Ramos S. R., Trabulsi L. R. Enteropathogenic Escherichia coli serotypes and endemic diarrhea in infants. Infect Immun. 1983 Feb;39(2):586–589. doi: 10.1128/iai.39.2.586-589.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabulsi L. R., Camargo M. E. Comparative study between immunofluorescence and coproculture in the diagnosis of intestinal infections by enteropathogenic Escherichia coli. Rev Inst Med Trop Sao Paulo. 1965 Mar-Apr;7(2):65–71. [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Vaith P., Uhlenbruck G. The Thomsen agglutination phenomenon: a discovery revisited 50 years later. Z Immunitatsforsch Immunobiol. 1978;154(1):1–15. [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]






