Abstract
Bone-eating worms of the genus Osedax colonized and grew on cow bones deployed at depths ranging from 385 to 2893 m in Monterey Bay, California. Colonization occurred as rapidly as two months following deployment of the cow bones, similar to the time it takes to colonize exposed whalebones. Some Osedax females found on the cow bones were producing eggs and some hosted dwarf males in their tubes. Morphological and molecular examinations of these worms confirmed the presence of six Osedax species, out of the eight species presently known from Monterey Bay. The ability of Osedax species to colonize, grow and reproduce on cow bones challenges previous notions that these worms are ‘whale-fall specialists.’
Keywords: Annelida, Polychaeta, Siboglinidae, whale-fall, cytochrome-c-oxidase subunit I
1. Introduction
Recent studies of submerged whale carcasses (whale-falls) have revealed a potentially worldwide distribution of bone-eating polychaete worms of the genus Osedax (Annelida, Siboglinidae; Rouse et al. 2004, in press; Glover et al. 2005; Dahlgren et al. 2006; Fujikura et al. 2006, 2007). Lacking a mouth and gut, Osedax females host heterotrophic bacterial symbionts (Oceanospirillales) in a branching ‘root’ system that invades the bone to extract organic compounds (Goffredi et al. 2005, 2007). A time-series analysis of whale carcasses deployed at varying depths in Monterey Bay, California, has revealed that exposed bones are colonized as rapidly as two months following their deposition on the seafloor (Braby et al. 2007). Such rapid colonization of a spatially scattered and temporally unpredictable food resource requires an immense pool of propagules, which disperse as fertilized eggs and developing larvae, though their location in the water column is presently unknown. Indeed, genetically based estimates of Osedax population sizes are large and comparable with those of other deep-sea polychaetes (Rouse et al. 2004, in press).
Osedax have been called ‘whale worms’ and ‘whale-fall specialists’ by several researchers (Glover et al. 2005; Dahlgren et al. 2006; Fujikura et al. 2006). Historically, the large lipid-rich bones of whales (Smith & Baco 2003) have undoubtedly provided a substantial food resource for Osedax, but the capacity of Osedax to grow and reproduce on other mammalian and possibly non-mammalian bones has not been studied experimentally. Here we report our efforts during the past 2 years to test whether Osedax are whale-fall specialists by deploying cow bones (an unlikely resource for marine worms) at depths ranging from 385 to 2893 m in Monterey Bay, CA.
2. Material and methods
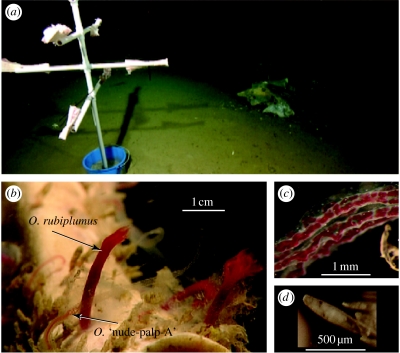
Fresh bovine femurs (20–26 cm long) with most of the flesh removed were cut in half longitudinally and suspended with cable ties from ‘bone trees’ constructed from 1.9 cm diameter PVC pipe (figure 1a). Each bone tree holding six bones was anchored in a plastic bucket filled with 7 kg of concrete. Altogether seven trees were deployed prior to the writing of this report. We used the robotic submersibles ROV Tiburon and ROV Ventana to deploy bone trees adjacent to Monterey Bay whale-falls at four depths (table 1). The biological communities found at each whale-fall at the time of these deployments were described elsewhere (Braby et al. 2007). The cow bones were closely scanned with high definition video (figure 1b) during subsequent submersible visits and sampled if Osedax females were present (table 1). Individual worms were dissected from the recovered bones and positively identified by their species-diagnostic mitochondrial cytochrome-c-oxidase subunit I (mtCOI) sequences. DNA extractions, PCR amplifications and DNA sequencing followed established protocols for Osedax (Goffredi et al. 2005; Braby et al. 2007). Unique mtCOI sequences produced in this study have been deposited in GenBank (table 3).
Figure 1.
(a) Deployed ‘bone tree’ adjacent to whale-2893 during May 2006. (b) In situ close-up from high definition video of Osedax rubiplumus and O. nude-palp-A on cow bone adjacent to whale-1820. (c) Close-up of the four palps from O. nude-palp-A showing paired blood vessels in each palp and absence of pinnules. (d) Dwarf (paedomorphic) males isolated from the tube of O. rubiplumus specimen shown in (b).
Table 1.
Osedax species found on cow bones in Monterey Bay, CA.
| whale-depth (m) | bone tree deployed | prior visits in daysa | bone recovered | days to recovery | diveb | latitude N | longitude W | proximity to carcass (m) | Osedax species (no. of worms)c |
|---|---|---|---|---|---|---|---|---|---|
| whale-385 | 25 Jan 2006 | 120, 272 | 11 Jan 2007 | 351 | T1070 | 36.79019928 | 121.8872376 | 1.2 | 0 |
| 25 May 2006 | 128, 231 | 4 Jun 2007 | 375 | V3014 | 36.79017639 | 121.8872681 | 0.8 | yellow-collar (7) | |
| whale-1018 | 9 Nov 2005 | 0 | 4 Jan 2006 | 56 | T931 | 36.77140427 | 122.0829163 | 1.7 | rosy (2) |
| 25 May 2006 | 56, 63, 78 | 25 Oct 2006 | 153 | T1049 | 36.77149963 | 122.0830536 | 2.4 | rosy (3) | |
| 79, 153 | 12 Jan 2007 | 233 | T1072 | 36.77149963 | 122.0830536 | 2.4 | 0 | ||
| whale-1820 | 23 May 2006 | 0 | 24 Oct 2006 | 154 | T1048 | 36.70830154 | 122.1050644 | 0.9 | nude-palp-A (1)nude-palp-A (3) |
| 12 Jan 2007 | 0 | 7Aug 2007 | 207 | T1119 | 36.70837900 | 122.1052120 | 1.7 | rubiplumus (2) frankpressi (1) | |
| whale-2893 | 24 May 2006 | 0 | 10 Jan 2007 | 231 | T1069 | 36.61341300 | 122.4354320 | 9.2 | nude-palp-B (3) |
Days post-deployment that bones were observed prior to recovery.
Submersible dive numbers: T indicates ROV Tiburon; V indicates ROV Ventana.
Zero (0) indicates no Osedax detected on recovered bone; negative sign (−) indicates none seen in video.
Table 3.
Mean sequence divergence within (diagonal) and between (lower left) described and undescribed Osedax species. (Total DNA sample sizes including redundant sequences (N) and GenBank accession numbers for unique sequences are given for each species. Published DNA sequences from Osedax japonicus from Japan and O. mucofloris from Sweden are considered for comparative purposes.)
| Osedax species | rubi. | fran. | muco. | japo. | rosy | yell. | oran. | spir. | nudA. | nudB. | N | GenBank acc. nos.a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rubiplumus | 0.39 | 32 | DQ996616-20, EU223297-311 | |||||||||
| frankpressi | 18.38 | 0.50 | 16 | DQ996621, EU223312-16 | ||||||||
| mucofloris | 20.73 | 17.28 | 0.55 | 10 | AY827562-68 | |||||||
| japonicus | 20.17 | 19.20 | 12.95 | n.c. | 1 | AB259569 | ||||||
| rosy | 20.01 | 19.27 | 16.77 | 14.32 | 0.11 | 25 | DQ996625-28, EU032469-84, EU223317-19 | |||||
| yellow-collar | 19.33 | 17.09 | 14.99 | 10.74 | 16.34 | 0.33 | 34 | DQ996629-38, DQ996640, EU223320-38 | ||||
| orange-collar | 22.41 | 15.87 | 16.84 | 11.67 | 21.28 | 6.10 | 0.35 | 21 | DQ996639, DQ996641-43, EU223339-55 | |||
| spiral | 25.32 | 24.71 | 22.42 | 22.76 | 23.63 | 23.84 | 27.07 | 0.00 | 5 | DQ996622-24 | ||
| nude-palp-A | 19.13 | 19.43 | 16.14 | 17.07 | 15.64 | 13.94 | 18.86 | 24.06 | 0.41 | 4 | EU223356-59 | |
| nude-palp-B | 28.52 | 17.99 | 24.72 | 18.98 | 14.90 | 19.14 | 18.96 | 25.85 | 17.90 | n.c. | 1 | EU236218 |
Sequences new to this study are reported in italics.
3. Results
To date, morphological and molecular analyses have identified eight species of Osedax from Monterey Bay. Their currently known depth range, distinguishing features and sequence divergence are summarized in tables 2 and 3. Osedax rubiplumus (Rouse et al. 2004) and Osedax frankpressi (Rouse et al. 2004) were the first species described in this genus, and a formal description of Osedax ‘rosy’ will soon appear (Rouse et al. in press). Three additional species (Osedax ‘yellow-collar’, O. ‘orange-collar’ and O. ‘spiral’) were briefly characterized by Braby et al. (2007) but remain unnamed. In this study, we identified two putatively new species, Osedax ‘nude-palp-A’ from 1820 m depth and O. ‘nude-palp-B’ from 2893 m depth. They are the only known Osedax species with four anterior palps that lack the characteristic feathery pinnules found on most Osedax. Instead, the palps of O. nude-palp-A and O. nude-palp-B are thin cylinders that contain two major blood vessels (figure 1c). Otherwise, females of the two species resemble other known Osedax species in having dwarf males. Though externally similar, the mtCOI sequences of O. nude-palp-A and O. nude-palp-B differ by almost 18%, and they differ by at least 14% from all other known species from Monterey Bay and elsewhere (table 3). Pairwise sequence divergences among these 10 putative species greatly exceeds intraspecific levels of sequence divergence (less than 1%), though only single populations of each named species has been examined to date. We did not provide a phylogenetic analysis based on presently available mtCOI sequences because the resulting trees are poorly resolved at deeper nodes. The present sequences, which include the 5′-end of mtCOI, are provided for DNA bar coding purposes (Hebert et al. 2003) and intended to facilitate the identification of Osedax species as they are discovered.
Table 2.
Described and undescribed Osedax species found in Monterey Bay, California.
| Osedax species | depths (m) | sizea | palps (colour) | pinnules (orientation) | roots | references |
|---|---|---|---|---|---|---|
| rubiplumus | 1820–2893 | 59 | 4 (brilliant red) | yes (outward) | long, branched | Rouse et al. (2004) |
| frankpressi | 1820–2893 | 23 | 4 (red with white stripe) | yes (inward) | robust, lobate | Rouse et al. (2004) |
| rosy | 633–1820 | 24 | 4 (red) | yes (outward) | long, branched | Rouse et al. (in press) |
| yellow-collar | 385–1018 | ∼18 | 4 (pale) | yes (in & out) | robust, lobate | Braby et al. (2007) |
| orange-collar | 385 | ∼18 | 4 (pale) | yes (undefined) | robust, lobate | Braby et al. (2007) |
| spiral | 2893 | ∼25 | none | no | filamentous | Braby et al. (2007) |
| nude-palp-A | 1820 | ∼25 | 4 (red) | no | unknown | this report |
| nude-palp-B | 2893 | ∼25 | 4 (red) | no | unknown | this report |
Maximum length of trunk and crown (mm) when preserved.
Six out of the eight Monterey species of Osedax have colonized experimentally deployed cow bones (table 1) and correspond with species found on the adjacent whale-fall. Osedax yellow-collar and orange-collar are found on whale-385, but only yellow-collar has colonized nearby cow bones. Osedax rosy is abundant on whale-1018 and on adjacent cow bones. Two Osedax species (O. rubiplumus and O. frankpressi) occur on whale-1820 and both occur on adjacent cow bones. Osedax nude-palp-A has only been observed on cow bones adjacent to whale-1820. Since discovering whale-2893, a natural carcass, in 2002 we have observed O. rubiplumus, O. frankpressi and O. spiral living there (Braby et al. 2007). But Osedax are scarce now on its highly degraded bones, and these species have not been observed on adjacent cow bones. Nonetheless, three specimens of Osedax nude-palp-B were found on an adjacent cow bone. Though Osedax species nude-palp-A and nude-palp-B were not observed on adjacent whalebones, these transparent worms might have been overlooked among the dense colonies of O. rubiplumus and O. frankpressi on whale-1820 and whale-2893, respectively. Both of the undescribed ‘nude-palp’ species were subsequently collected from whalebones following detailed video surveys of whale-1018.
Colonization and growth of Osedax on the deployed cow bones occurred rapidly at two depths. Osedax rosy was found 56 days post-deployment near whale-1018 (table 1) but the sampled worms did not contain eggs. Three months later, 154 days post-deployment, the worms had matured, as three individuals of Osedax rosy contained eggs. Similarly, a mature O. rubiplumus female (154 days post-deployment and adjacent to whale-1820) had eggs in her oviduct and a harem of dwarf (paedomorphic) males in her tube (figure 1d) that are a notable characteristic of this siboglinid genus (Rouse et al. 2004, in press). Colonization of cow bones at the shallowest site was markedly slower, about 1 year, but Osedax colonization of whale-385 was also slow, a probable consequence of frequent disturbance due to intense sediment flows in this part of Monterey Canyon (Braby et al. 2007). Colonization of cow bones near the deepest carcass, whale-2893 m, also was slow.
4. Discussion
By considering Osedax worms as whale-fall specialists, Glover et al. (2005, p. 2591) concluded that the size of ‘Osedax habitat is dependant on the current population densities of cetaceans that are known to create lipid-rich whalebone reefs.’ Large whalebones have the capacity to provide habitats that may persist for decades (Smith & Baco 2003; Schuller et al. 2004). But here we report on six Osedax species that can grow on smaller mammalian bones with significantly lower lipid contents than intact whalebones (Evershed et al. 1995; Goffredi et al. 2007). Each cow bone used for this study was cut longitudinally prior to deployment, and the exposed marrow had been removed by the time we sampled the bones several months later. All the Osedax that we found on cow bones were growing in hard lamellar bone. Clearly, the heterotrophic bacterial endosymbionts hosted by Osedax are capable of extracting diverse carbon sources, dominated by collagen and cholesterol, from hard bone (Goffredi et al. 2005). The Osedax root tissues housing these Oceanospirillales bacteria produce proteolytic enzymes that are capable of hydrolysing collagen, a resource that is otherwise difficult to digest (Goffredi et al. 2007). Furthermore, these bacteria can be maintained in cultures enriched in collagen and cholesterol (S. Goffredi 2007, personal communication). Thus, given our current knowledge of these nutritional characteristics, there is no reason to believe that Osedax should be restricted to large lipid-rich whalebones.
Females belonging to two of the Osedax species found growing on deployed cow bones were producing eggs, and one female had accumulated a harem of dwarf males, a general characteristic of Osedax (Rouse et al. in press). It is probable, therefore, that a variety of mammalian bones provide sufficient sources of nutrition for the growth and reproduction of Osedax. A broad range of small cetacean and pinniped bones probably contribute to the maintenance of Osedax populations in Monterey Bay (e.g. common dolphins, Delphinus delphis, harbour porpoises, Phocoena phocoena, northern elephant seals, Mirounga angustirostris, sea lions, Zalophus californianus, and harbour seals, Phoca vitulina). Cows and other terrestrial quadrupeds probably do not provide regular food sources for Osedax, but native and domesticated ungulates are abundant in the flood plains of coastal rivers and their carcasses will probably be found in storm debris that settles in the submarine canyons. Finally, we cannot exclude the possibility that large fish bones and shark cartilage might also provide sources of collagen to support some fast-growing Osedax species. A problem with small bones and cartilage is that they are likely to be buried in sediments and unavailable to most species of Osedax; however, O. spiral may be an exception as it has only been found rooted in buried whalebone fragments (Braby et al. 2007). Clearly, similar experiments with a wide variety of vertebrate bones and carcasses are suggested by the present results, and we plan to carry them out during the next several years.
Acknowledgments
We thank the pilots of ROV Tiburon and ROV Ventana, and crews of RV Western Flyer and RV Point Lobos for their expert help. This work was supported by grants from the David and Lucile Packard Foundation.
References
- Braby C.E, Rouse G.W, Johnson S.B, Jones W.J, Vrijenhoek R.C. Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey Bay, California. Deep Sea Res. I. 2007;54:1773–1791. doi:10.1016/j.dsr.2007.05.014 [Google Scholar]
- Dahlgren T.G, Wiklund H, Kallstrom B, Lundalv T, Smith C.R, Glover A.G. A shallow-water whale-fall experiment in the north Atlantic. Cah. Biol. Mar. 2006;47:385–389. [Google Scholar]
- Evershed R.P, Turner-Walker G, Hedges R.E.M, Tuross N, Leyden A. Preliminary results for the analysis of lipids in ancient bone. J. Archaeol. Sci. 1995;22:277–290. doi:10.1006/jasc.1995.0030 [Google Scholar]
- Fujikura K, Fujiwara Y, Kawato M. A new species of Osedax (Annelida: Siboglinidae) associated with whale carcasses off Kyushu. Jpn Zool. Sci. 2006;23:733–740. doi: 10.2108/zsj.23.733. doi:10.2108/zsj.23.733 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, et al. Three-year investigations into sperm whale-fall ecosystems in Japan. Mar. Ecol. Evol. Perspect. 2007;28:219–232. doi:10.1111/j.1439-0485.2007.00150.x [Google Scholar]
- Glover A.G, Kallstrom B, Smith C.R, Dahlgren T.G. World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc. R. Soc. B. 2005;272:2587–2592. doi: 10.1098/rspb.2005.3275. doi:10.1098/rspb.2005.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffredi S.K, Orphan V.J, Rouse G.W, Jahnke L, Embaye T, Turk K, Lee R, Vrijenhoek R.C. Evolutionary innovation: a bone-eating marine symbiosis. Environ. Microbiol. 2005;7:1369–1378. doi: 10.1111/j.1462-2920.2005.00824.x. doi:10.1111/j.1462-2920.2005.00824.x [DOI] [PubMed] [Google Scholar]
- Goffredi S.K, Johnson S.B, Vrijenhoek R.C. Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. Appl. Environ. Microbiol. 2007;73:2314–2323. doi: 10.1128/AEM.01986-06. doi:10.1111/j.1462-2920.2005.00824.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert P.D.N, Cywinska A, Ball S.L, deWaard J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. doi:10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse G.W, Goffredi S.K, Vrijenhoek R.C. Osedax: bone-eating marine worms with dwarf males. Science. 2004;305:668–671. doi: 10.1126/science.1098650. doi:10.1126/science.1098650 [DOI] [PubMed] [Google Scholar]
- Rouse, G. W., Worsaae, K., Johnson, S. B., Jones, W. J. & Vrijenhoek, R. C. In press. Acquisition of dwarf male ‘harems’ by recently settled females of Osedax ‘rosy’ n. sp. (Siboglinidae; Annelida). Biol. Bull [DOI] [PubMed]
- Schuller D, Kadko D, Smith C.R. Use of 210Pb/226Ra disequilibria in the dating of deep-sea whale falls. Earth Planet. Sci. Lett. 2004;218:277–289. doi:10.1016/S0012-821X(03)00690-3 [Google Scholar]
- Smith C.R, Baco A.R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:311–354. [Google Scholar]