Abstract
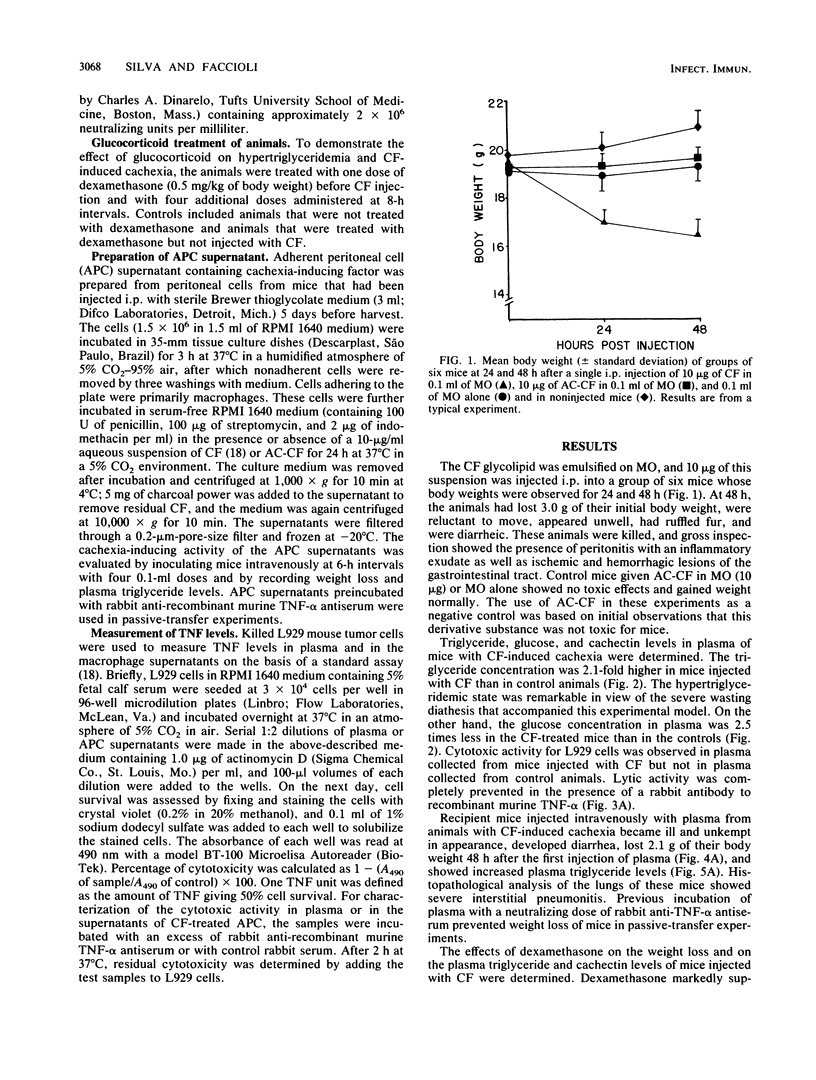
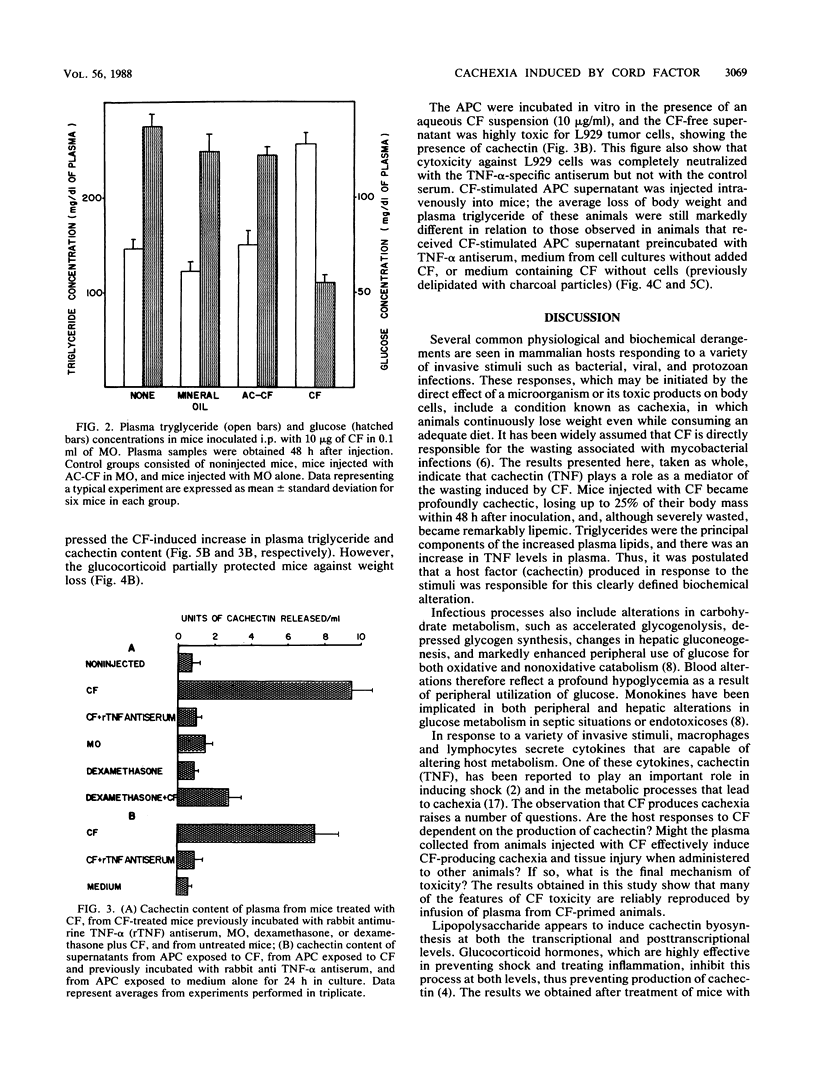
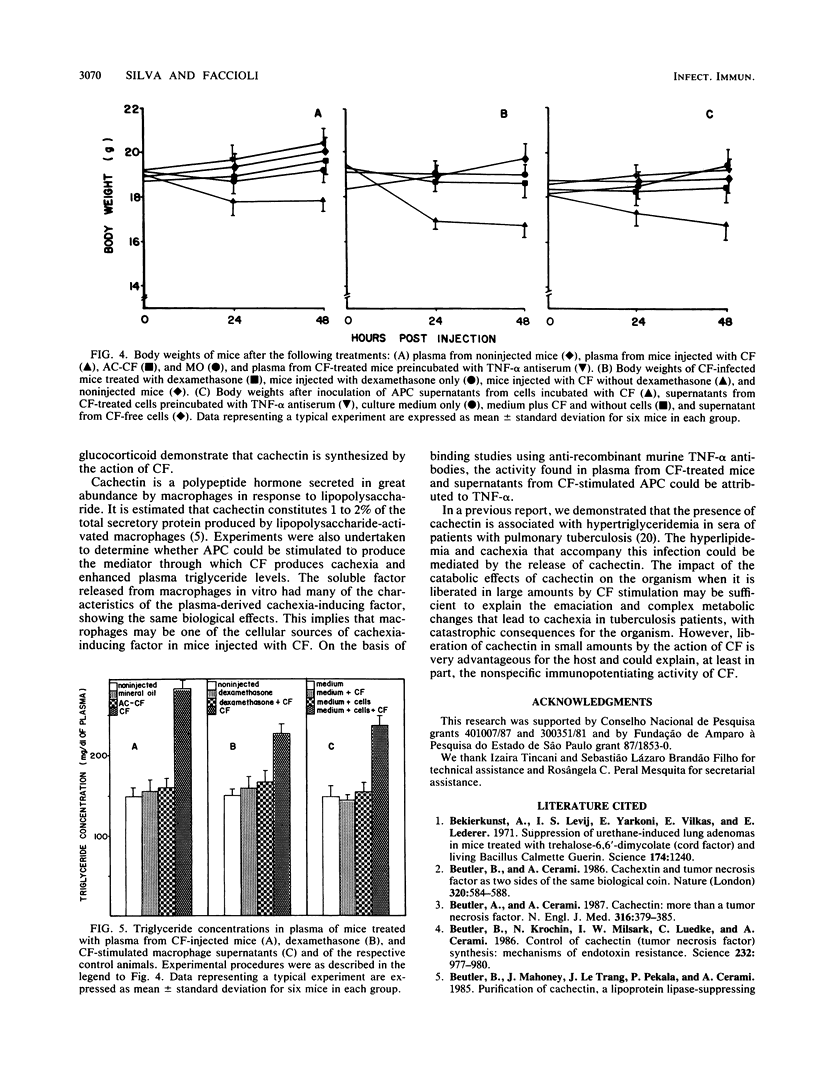
The mechanism by which cord factor (CF), a toxic glycolipid from mycobacteria, induces cachexia was studied in BALB/c mice. Body weight was markedly reduced 48 h after CF administration; the animals became severely wasted and exhibited hypertriglyceridemia, hypoglycemia, and high levels of tumor necrosis factor (TNF) in plasma. After CF administration, a transferable factor which caused cachexia and hypertriglyceridemia in recipient mice was detected in the blood. Dexamethasone partially inhibited the cachexia-inducing action of CF. Conditioned medium from adherent peritoneal cell cultures incubated with CF produced the same wasting symptoms when inoculated intravenously into mice. These studies also demonstrated that adherent peritoneal cells produced a humoral factor in response to CF which was related to CF-induced cachexia. Antiserum to recombinant TNF-alpha prevented the cachectin action in passive-transfer experiments. Our findings indicate that cachectin (TNF) plays a role as a central mediator of the wasting induced by CF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E. Suppression of urethan-induced lung adenomas in mice treated with trehalose-6,6-dimycolate (cord factor) and living bacillus Calmette Guérin. Science. 1971 Dec 17;174(4015):1240–1242. doi: 10.1126/science.174.4015.1240. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkins J. P. Monokines and the metabolic pathophysiology of septic shock. Fed Proc. 1985 Feb;44(2):300–304. [PubMed] [Google Scholar]
- Hotez P. J., Le Trang N., Fairlamb A. H., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by a haematoprotozoan-induced mediator from peritoneal exudate cells. Parasite Immunol. 1984 May;6(3):203–209. doi: 10.1111/j.1365-3024.1984.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Kato M., Asselineau J. Chemical structure and biochemical activity of cord factor analogs. 6,6'-Dimycoloyl sucrose and methyl 6-mycoloyl- -D-glucoside. Eur J Biochem. 1971 Oct 14;22(3):364–370. doi: 10.1111/j.1432-1033.1971.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Minnikin S. M., Dobson G., Goodfellow M., Portaels F., van den Breen L., Sesardic D. Mycolic acid patterns of four vaccine strains of Mycobacterium bovis BCG. J Gen Microbiol. 1983 Mar;129(3):889–891. doi: 10.1099/00221287-129-3-889. [DOI] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Drapier J. C., Petit J. F., Wietzerbin J., Lederer Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6'-trehalose dimycolate). J Infect Dis. 1977 May;135(5):771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A., Cerami A. Hypertriglyceridemia associated with Trypanosoma brucei brucei infection in rabbits: role of defective triglyceride removal. Mol Biochem Parasitol. 1980 Oct;2(1):31–38. doi: 10.1016/0166-6851(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Senn M., Ioneda T., Pudles J., Lederer E. Spectrométrie de masse de glycolipides. I. Structure du "cord factor" de Corynebacterium diphtheriae. Eur J Biochem. 1967 May;1(3):353–356. doi: 10.1111/j.1432-1033.1967.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Silva C. L., Faccioli L. H., Rocha G. M. The role of cachectin/TNF in the pathogenesis of tuberculosis. Braz J Med Biol Res. 1988;21(3):489–492. [PubMed] [Google Scholar]


