Abstract
Tachykinin NK3 receptor (NK3R) is a g-protein coupled receptor that is heavily expressed by magnocellular neurons of the paraventricular nucleus of the hypothalamus (PVN). Osmotic challenge is reported to activate NK3R expressed by magnocellular neurons and cause the NK3R to be internalized to the cytoplasm and perhaps the cell nucleus. In this study we show using immuno-electron microscopy that isolated nuclei from neurons in the PVN of osmotic challenged animals show a robust labeling for the NK3R. NK3R immunoreactivity was detected by Western blot in isolated nuclei of PVN neurons following the 2 M NaCl injection. No nuclear NK3R immunoreactivity was detected in control animals. NK3R antibody specificity was confirmed by small interfering (SI) RNA technology. This study establishes that the NK3R is trafficked to the nucleus of PVN neurons following a peripheral osmotic challenge.
Keywords: G-protein coupled receptor, Cell signaling, Electron microscopy, siRNA
Magnocellular neurons of the hypothalamus synthesize and secrete two key hormones: vasopressin (VP) and oxytocin (OT). The release of these hormones is influenced by a number of transmitters and magnocellular neurons express a number of receptors. Noteworthy is the tachykinin, NK3 receptor (NK3R) which is heavily expressed by magnocellular neurons of the supraoptic (SON) and paraventricular nucleus (PVN) of the hypothalamus (Ding et al., 1999). NK3R expressed by magnocellular neurons appears to have a functional role in the release of vasopressin and oxytocin. Stimulation of NK3R results in an increase in Ca++ conductance (Buell et al., 1992), induction of c-Fos expression in magnocellular neurons, and causes the systemic release of both hormones (Ding et al., 1999; Smith and Flynn, 2000; Bealer and Flynn, 2003). Furthermore, the release of the endogenous ligand, presumably neurokinin B (NKB), and binding to the NK3R results in the internalization of the peptide-receptor complex (Chawala et al., 1997; Colin et al., 2002; Lessard et al., 2004; Johnson et al., 2004) that can be tracked immunohistochemically. NK3R expressed by magnocellular neurons are membrane bound in control (isotonic saline treated) animals. After hyperosmotic or hypotensive challenges, NKB is released locally because NK3R are internalized within organelles in the soma and dendrites of VP magnocellular neurons (Howe et al., 2004; Haley and Flynn, 2006). Interestingly, immunohistochemical results suggested that subsequent to the cytoplasmic sequestration of the NK3R, NK3R immunoreactivity was detected in the nuclei of magnocellular VP neurons in animals (Haley and Flynn, 2006); (Howe et al., 2004).
The conclusion that NK3R is trafficked to the cell nucleus is tempered by issues related to the specificity of the antibody and the optical limits of confocal laser scanning microscopes. There are established, alternative methods to quantify the nuclear accumulation of the receptor (Pickard et al., 2007) and establish the specificity of the antibody. First, isolation of subcellular fractions, particularly the nuclei of PVN neurons after the osmotic challenge, provides a precise way to confirm that the NK3R is transported to the nucleus following an osmotic challenge. Isolating the nuclei from these neurons assures that the NK3R that is detected is only from the nucleoplasm and not from internalized cytoplasmic receptors. Second, immuno-transmission electron microscopy provides the resolution necessary to determine if the NK3R enters the nucleoplasm or if the receptor is only associating with the nuclear membrane. Third, small interfering RNA (siRNA) technology is a means to validate or establish the specificity of the antibody. siRNA provides for the suppression of specific proteins based on the unique mRNA sequence. Although off target effects have been reported (Jackson et al., 2006), these effects can be controlled for by measuring the expression of housekeeping genes, like actin, and the use of control mismatched siRNA sequences. The following experiment details specificity of the NK3R antibody and the accumulation of NK3R protein in nuclei of PVN neurons following osmotic challenge in-vivo using immuno-electron microscopy and Western blot.
Experimental Procedures
Subjects and procedure
All animal experiments were carried out in accordance with the National Institute of Health guidelines for the care and use of laboratory animals and were approved by the University of Wyoming Animal Care and Use Committee. Male Charles River rats (300 g) were housed in individual hanging stainless steel cages and kept on a 12 hour light/dark cycle (N=67; n=45 for siRNA, n=12 for Nuclear Western blot, n=10 for TEM). Animals were given access to water and rat chow ad libitum. Animals were adapted to the intragastric tubing procedure on several days. On the test day, animals were removed from their cages and an infant feeding tube guided into their stomach. Animals were administered intragastric loads (IG) of either 2 M NaCl (6 ml) or a sham load delivered in about 1 minute. The feeding tube was then withdrawn and the animals returned to their home cages. At 40 minutes (n = 8) or 2 hours (n = 4) following the IG loading the animals were administered a lethal dose of pentobarbital sodium, decapitated, and the brains excised without PBS perfusion in under 3 minutes per brain. The brain was then rinsed with ice cold PBS and using a surgical microscope the brain was sectioned at the bifurcation of the optic tract and again 2 mm posterior to the first cut. The PVN was dissected free of the surrounding tissue providing a tissue block that contained both the parvocellular and magnocellular divisions of the PVN. In addition, the tissue contained portions of the anterior and medial preoptic nucleus; areas that are either devoid of the NK3R or only weakly label for NK3R (Ding et al., 1996). The tissue block contained Within the sample, NK3R are largely confined to magnocellular neurons, with limited expression of NK3R in the parvocellular PVN (Ding et al., 1996; Ding et al., 1999). The PVN tissue block was then homogenized using a Dounce homogenizer with 15–20 strokes of both the loose and tight pestles in 0.75 ml of homogenization buffer (0.32 M sucrose, 3 mM CaCl2, 2 mM MgCl2, 0.1 mM EDTA, 0.1% Triton X-100, 1 mM DTT, 10 mM Tris-HCl, pH 8.0) with Halt protease inhibitor (Pierce). The homogenate was transferred to a chilled microfuge tube and centrifuged at 1,000 × g for 15 minutes at 4° C. The supernatant was discarded and the pellet was resuspended in 1 ml of homogenization buffer and centrifuged a second time at 1,000 × g for 15 minutes at 4° C. The supernatant was again discarded and the pellet was resuspended in 0.75 ml of homogenization buffer. Next, 0.75 ml of sucrose buffer was added (1.8 M sucrose, 5 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 10 mM Tris-HCl, pH 8.0) and vortexed 15 seconds. The resulting mixture was overlaid on 1.0 ml of sucrose buffer in a pre chilled clean centrifuge tube. The nuclei were pelleted through the sucrose buffer cushion at 30,000 × g for 1 hour at 4° C in a swinging bucket rotor. The supernatant was carefully removed to avoid contamination of the nuclear pellet, and then discarded. The isolated nuclei were either fixed for transmission electron microscopy or lysed with 40 µl of 8 M urea and 40 µl of SDS sample buffer (3% SDS, 10 mM Tris, 1 mM EDTA, and 2% non-reducing lane marker sample buffer [Pierce Rockford, IL]).
Transmission Electron Microscopy
In the experiment NK3R immunogold labeling was quantified in isolated nuclei from the PVN tissue. Nuclei, rather than whole tissue, were used for several reasons. First, a number of studies have characterized the cytoplasmic transport of NK3R (Colin et al., 2002; Schmidlin et al., 2003; Howe et al., 2004; Haley and Flynn, 2006). Second, the scientific focus of the experiment was to provide detailed, new information on the nuclear transport of NK3R. Third, the nuclear fraction provided a greater number of nuclei per grid that can be quantified. This enables a greater sampling of nuclei from each PVN tissue block. Finally, the purity of the nuclear fractions (not contaminated by other cytoplasmic organelles) is essential for the interpretation of Western data. As shown below, the nuclear fractions contained nuclei and were free of other structural artifacts.
The isolated nuclei were re-suspended by drop-wise addition of PBS. The nuclei were then pelleted by centrifugation and the PBS was decanted. The nuclei were then fixed using 4% para-formaldehyde and 0.25% glutaraldehyde mixed in PBS for one hour at room temperature. The nuclear fractions were then washed and went to two different procedures. Nuclear fractions from non-challenged animals (n = 2) were used for nuclear ultrastructure and were additionally fixed in 2% osmium tetroxide for 30 min. The use of osmium tetroxide preserves the lipids in the membrane but destroys the antigenicity of the sample. Nuclear fractions used for the immunogold labeling (n = 8) of NK3R were fixed with 4% para-formaldehyde and 0.25% glutaraldehyde only. The lack of osmium tetroxide allows the samples to retain the antigenicity but the preservation of the membranes is not as complete. Following fixation, the nuclei were washed in dH2O and were then dehydrated by using a graded ethanol wash (50% x1, 70% x1, 85% x1, 95% x1, 100% x3), 5 min. each. The nuclei were embedded in LR White resin (London Resin Company, Berkshire, UK) and allowed to polymerize at 60° C for 24 hours. The resin blocks containing the nuclei were prepared and 50 nm sections were cut using the ultra microtome. The 50 nm sections containing nuclei were placed onto formvar coated nickel mesh grids (Electron Microscopy Sciences, Hatfield, PA) for support. Tissue sections were processed for the presence of the NK3R in the nucleus by immunogold staining. Grids containing the sectioned nuclei were first treated with 0.1 M glycine in PBS to inactivate residual aldehydes. The grids were then washed in PBS containing 0.1% Triton X-100 for 5 minutes. Grids then were floated on blocking solution consisting of PBS with 0.2% Tween-20, 0.2% BSA-C and 5% normal goat serum for 40 minutes. Following the blocking step the grids were floated on incubation buffer (PBS pH 7.4, 0.2% Tween-20, 0.2% BSA-C) containing the K7 (1:25) antibody for 2 hours. The grids were washed twice in pure incubation buffer for 5 minutes and then in placed in blocking solution for another 20 minutes. Next, the grids were floated on incubation buffer containing the goat anti rabbit secondary 12 nm gold conjugated goat anti rabbit secondary antibody (1:30, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hours. The grids were washed with pure incubation buffer for 5 minutes and twice more in PBS for 5 minutes each. The grids were fixed for one hour with 8% glutaraldehyde and then washed 3 times in double distilled H2O for 5 minutes each. Each grid was then stained for 30 minutes in 2% uranyl acetate, washed, and then stained again for 15 seconds in lead citrate. The grids were washed, allowed to dry, and then viewed on a Hitachi H-7000 Electron Microscope (Pleasanton, CA). To assure an accurate sampling of the isolated nuclei, 5 nuclei per grid, and 5 grids per animal, for a total of 25 nuclei per animal and 4 animals per group where imaged. Electron micrographs were taken with a Gatan high resolution 4 k × 4 k digital camera and Gatan DigitalMicrograph software (Pleasanton, CA). The nuclear micrographs were stitched together with Adobe Photoshop version Cs2 (San Jose, CA) to provide a high resolution image of the entire nucleus. Nuclear gold beads were counted two ways. ImageJ (NIH) was calibrated to detect the 12 nm gold beads and used to quantify number of beads present in a nucleus. Lab technicians blind to the experimental manipulation also counted the numbers of gold beads present in the isolated nuclei. The number of gold beads counted within a nucleus never varied by more than ±3 gold beads between methods. ImageJ software (NIH) was also used to determine the surface area of the nuclei. Quantification of the nuclear immunoreactivity of the NK3R was determined by dividing the number of gold beads by the area of the nucleus (Beads/µm2). Background immunogold labeling was determined by counting the number of gold beads in areas of nuclei-free resin that was comparable in size to the nuclei (n = 95 for hypertonic saline animals, n = 53 for control) and then dividing that number of beads by the area of nuclei free resin (Beads/µm2).
siRNA
siRNA was used to confirm that the NK3R antibody was recognizing NK3R protein. siRNA provides high specificity of target suppression; suppression of protein translation is highly selective for the target protein with minimal nonspecific suppression of other proteins (Hamada et al., 2002; Schwarz et al., 2006). The siRNA construct was designed by MolelculA research Laboratories (Columbia MD). The sequence was also a top scoring sequence generated by the Sfold siRNA design software (Ding and Lawrence, 2005) and had no homology to any other eukaryotic RNA sequence (siRNA sequence 5′- C-GAU-CUA-CCA-ACA-GUU-A:AA-TTX-3′; 5’–UUU-AAC-UGU-UGG-UAG-AUC-GTT-3’). The siRNA sequence targets amino acids 310–316, located on the third extracellular loop of the receptor. A mismatched siRNA sequence was developed that contained the same GC% as the experimental siRNA construct but was not homologous to any known eukaryotic RNA sequence (Mismatched siRNA 5′-CUA-CUA-CUA-CUA-CAG-AGA-ATTX-3′; 5’-UUC-UCU-GUA-GUA-GUA-GUA-GTT-3’).
To transfect the PVN with the siRNA, male, Charles River rats (250–350 g, N=45; n = 6 siRNA and n=3 mismatched siRNA animals per time point) were anesthetized with ketamine (0.7 ml/kg) and placed in a stereotaxic instrument. The skull was exposed and a hole was drilled in to the skull 1.6 mm posterior to Bregma and 0.2 mm lateral to the midline. A glass micropipette was lowered 8.5 mm ventral to the skull to access the right PVN region. These coordinates were established by pilot studies and previous experiments, and these coordinates yield consistent injection results. The pipette was left in place for 15 minutes. Following the 15 minutes 250 nl (3.45 µg) of the siRNA construct or the mismatched control siRNA in sterile RNase free water was injected over 2 minutes. The pipette was left alone for 10 minutes and then removed. The wound was sutured and the animals were allowed to recover from the surgery. Animals were sacrificed at different time points to determine the time course for the knockdown and recovery of the NK3R. At the assigned time animas were given a lethal dose of pentobarbital sodium and decapitated. The brains were removed and rinsed in ice cold PBS. The PVN area was blocked using the procedure previously described. The tissue was then divided by cutting down the third ventricle to separate the noninjected PVN from the transfected PVN. The PVN rich tissue block was homogenized in RIPA buffer (Pierce, Rockford, IL) and the protein concentration was determined by photo-absorbance. Western blot analysis was used to determine knockdown of the NK3R. Each animal yielded ample protein for western blot analysis of both the transfected and noninjected PVN.
Western Blot Assay
Nuclear fractions from the IG loaded animals (n = 4 per group per treatment) and the siRNA transfected animals were run under the same western blot protocols. Isolated nuclei from hypertonic saline IG loaded animals were run next to the control isolated nuclei. For the siRNA experiment samples from the transfected PVN were ran next to samples from the contralateral noninjected PVN. Samples (40 µg/lane) were loaded on a 4–15% SDS polyacrylamide gel along with TriChromeRanger Marker (Pierce, Rockford, IL) and electrophoresed at a constant 200 volts. The samples were then transferred to PVDF membrane for 60 minutes at a constant 100 volts. Subsequently, the membrane was washed in TBST (Tris buffered saline, 0.05% Tween 20) and blocked for 1 hour in blocking buffer (TBST, 5% Blotto [BioRad, Hercules, CA]). The membrane was then incubated with primary antibody in blocking buffer for 1 hr at room temperature. Samples probed for the NK3R protein used an antibody (K7) directed against COOH terminus, amino acids 434–452 (kindly provided by Dr. James Krause; (Mileusnic et al., 1999)). The K7 antibody was used at a 1:4000 dilution for the immunoblot staining. The K7 antibody was pre-absorbed to show specificity for the amino acid sequence of the C-terminus of the NK3R peptide (Figure 1-D). The membrane was then washed 4 × 4 minutes at room temperature in TBST. Next the membrane was incubated with secondary antibody (Pierce stabilized goat anti-rabbit HRP at 1:5000) for 1 hour and subsequently washed 5 × 4 minutes at RT in TBST. The protein band was visualized using either the SuperSignal West Femto kit or SuperSignal West Pico kit (Pierce, Rockford, IL) depending on the target protein. Images were optically scanned (Hewlitt-Packard 5200C with HP PrecisionScan software v2.02, Palo Alto, CA) and digitized. Antigen signal was quantified by pixel density using arbitrary pixel units (Un Scan It gel v5.1; Silk Scientific, Orem, UT).
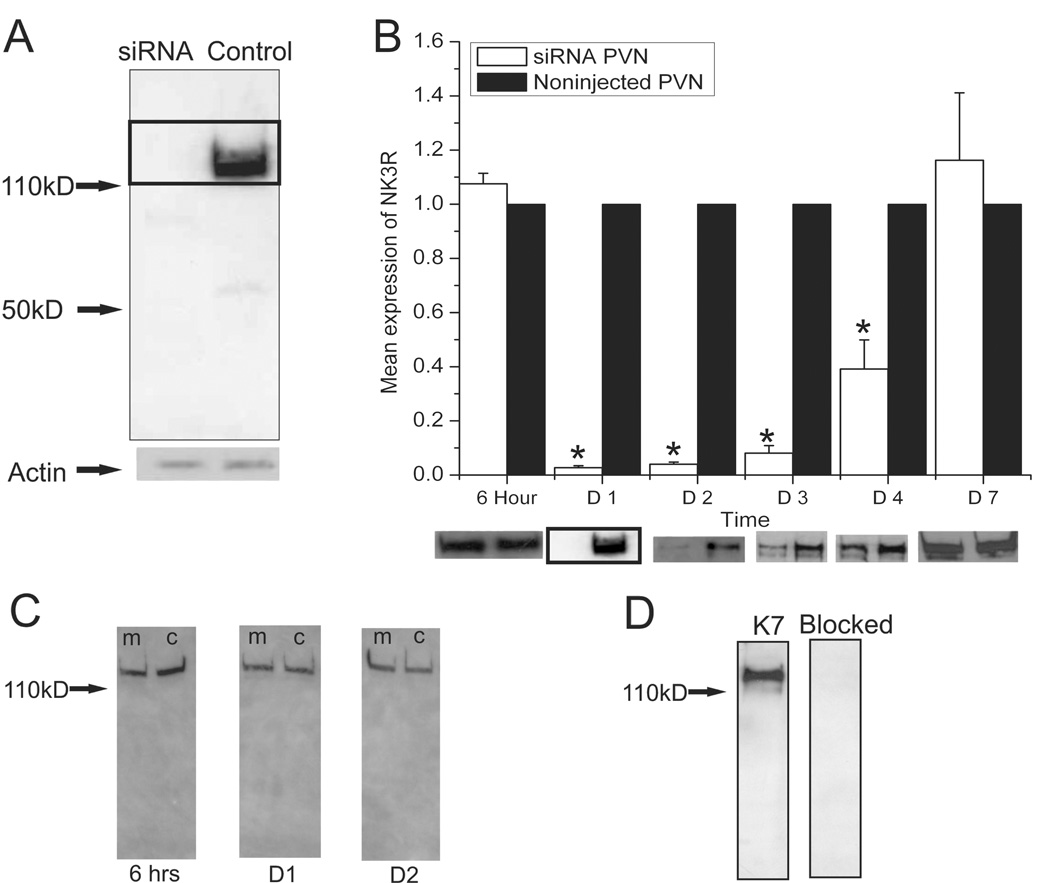
Figure 1.
(A) Western blot for NK3R showing a reduction of the receptor 24 hours following the injection of the siRNA. Levels of actin were not affected by the transfection with siRNA. (B) Quantification of western blot labeling for the NK3R following the injection of the siRNA as compared to the noninjected, contralateral PVN (Control). The siRNA caused a remarkable decrease in the expression of NK3R in the injected PVN as compared to the contralateral noninjected PVN in the same animals. The expression of NK3R gradually recovered until it reached normal expression levels seven days after the injection. Western blots showing the levels of NK3R at the different time points of the experiment. Note the boxed images is the same image seen in (A). (n= 6 per group, * P’s < 0.0001, one-way ANOVA) Error bars indicate SEM. (C) Western blots showing that the immunoreactivity for the NK3R was not affected by the mismatched siRNA. In the image (m) marks the transfected PVN and (c) marks the noninjected contralateral PVN for the animal. (D) The immuno-labeling of the NK3R was blocked by the pre-absorption of the K7 antibody with the antigenic peptide.
As a control for protein loads in the nuclear isolations each western blot was probed with an antibody for the nuclear histone H1 (0.4 µg/ml, Santa Cruz). The density of this histone band was used to normalize the amount of protein present in the different western blots and to compare control animals to salt challenged animals.
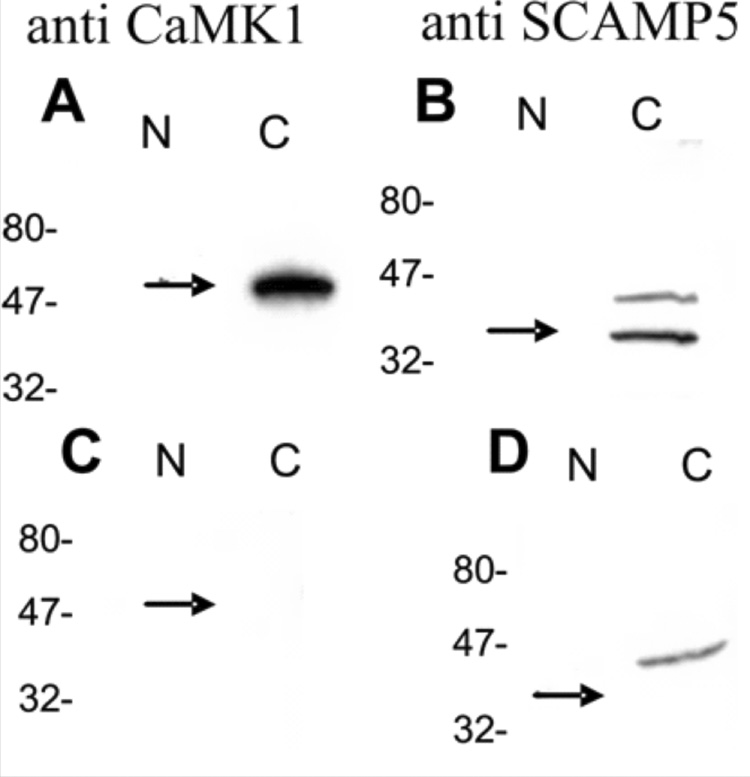
The purity of the nuclear preparation was evaluated (Figure 2) by probing the samples for the presence of two cytoplasmic proteins: SCAMP-5 (Hubbard et al., 2000) and CaMK1 (Naruse et al., 1985; Saavedra et al., 1986). Affinity purified antibodies against SCAMP5 and CaMK1 (Affinity Bioreagents, Golden, CO) were diluted to a working concentration of 2 µg/ml. The secondary antibody (chicken anti-rabbit) was diluted to 1:5000. Any nuclear samples that were contaminated by cytoplasmic proteins were discarded.
Figure 2.
Western blots showing the purity of the isolated nuclear sample. (A) Detection of the cytoplasmic protein CaMK1 in the cytoplasm and not within the nuclear sample. (B) Detection of the cytoplasmic protein SCAMP5 in the cytoplasm but not in the nuclear samples. (C, D) preabsorption of the CaMK1 and SCAMP5 antibodies.
Results
siRNA
siRNA is a effective tool in inhibiting the production of proteins in-vivo. The western blots of the siRNA injected PVN and the contralateral noninjected PVN were compared to see if the siRNA was effective in inhibiting the translation of NK3R. NK3R was readily detected by western blot analysis. The size of the immunoreactive band for the NK3R is larger than the weight predicted based on the amino acid sequence of the receptor. This larger band can be explained by the fact that GPCRs are known to form functional dimers that are resistant to denaturing in SDS PAGE gels (Overton and Blumer, 2000; Thevenin et al., 2005). A reduction in immunoreactive band density for NK3R was not seen 6 hours following the injection of siRNA, there was however a virtual elimination of the protein within the PVN 24–48 hours after the injection of the siRNA construct (Figure 1-A, B) as compared to the contralateral noninjected PVN of the same animal. During this time frame, the siRNA effectively inhibited the expression of NK3R and kept protein levels below the detectable limits of the assay. As shown (Figure 1-A) no bands were detected by the NK3R antibody. The expression of NK3R gradually recovered until it reached normal expression levels seven days following the siRNA injection. Injections of a mismatched siRNA construct failed to show any decrease in the expression of the NK3R at any of the time points during the experiment (Figure 1-C). At 6 hours following the mismatched siRNA injection there was 103% expression of NK3R as compared to the contralateral noninjected PVN. There was 109% and 101% expression of NK3R at 24 and 48 hours following the mismatched siRNA injection respectively. These values do not represent a significant change in the expression of NK3R due to the mismatched siRNA. β-actin was used as a control protein to normalize protein loads in the westerns and to better identify possible nonselective protein inhibition due to the siRNA injections. β-actin also provided a control to test for possible cytotoxicity effects of the siRNA transfection. There was no effect of siRNA on actin; throughout the experiment the β-actin levels did not change in any of the groups. The β-actin data along with the mismatched siRNA data demonstrate that the knockdown of the NK3R was due to direct effects of the siRNA blocking the production of NK3R and not due to some nonspecific affect of the siRNA construct. These results show that the protein detected by the K7 antibody is the same protein that is inhibited by our siRNA which is the NK3R.
Nuclear accumulation of NK3R
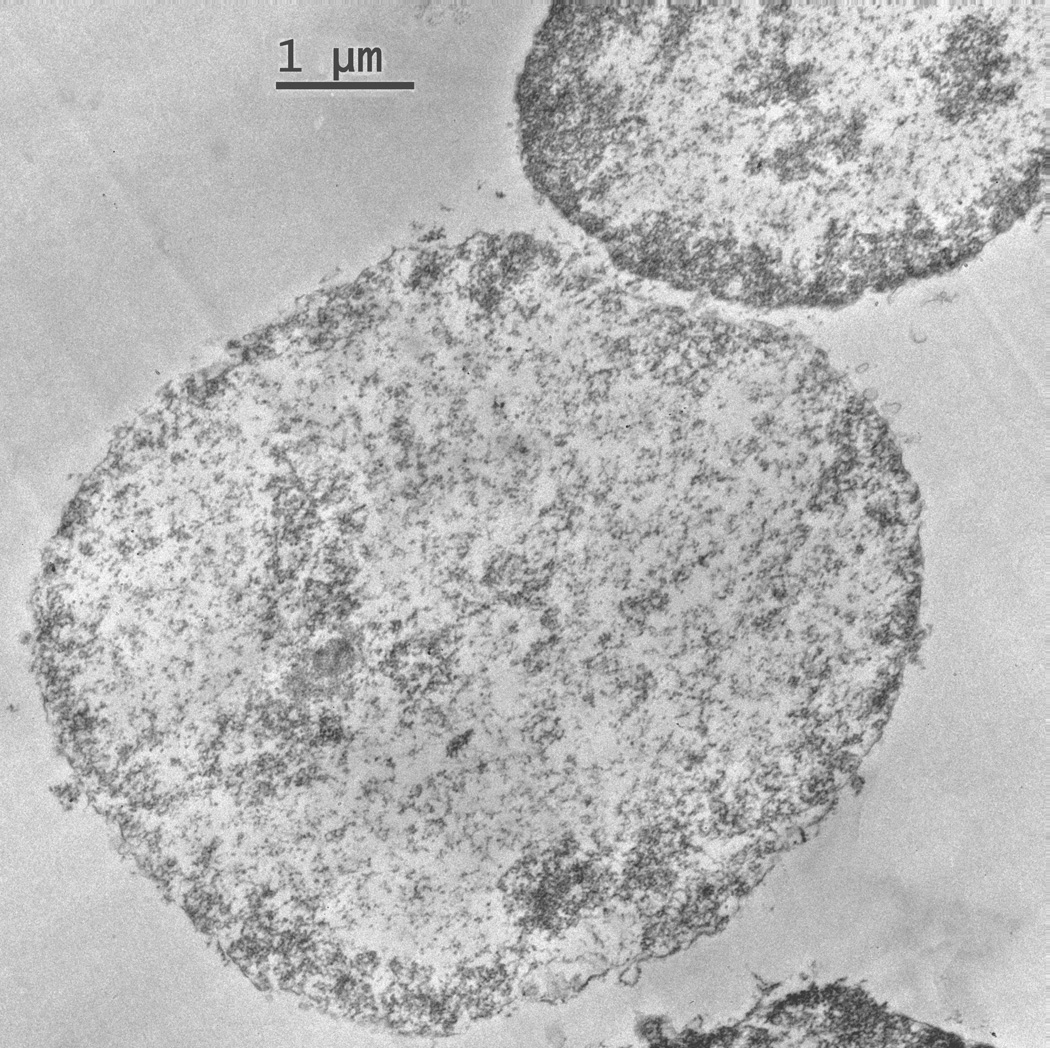
Electron micrographs of isolated nuclei that received the full fixation from the osmium tetroxide provide detail of the nuclear ultrastructure (Figure 3). Nuclear organelles, including the nuclear membrane and the nucleolus are identifiable in the micrographs. These micrographs show that the nucleus is intact and that there are not any perinuclear organelles, (i.e. Golgi or endoplasmic reticulum) attached to the isolated nuclei. This evidence is important for interpreting the Western analyses because it shows that any NK3R band is protein that has been trafficked to the nucleus and not protein associated with perinuclear organelles.
Figure 3.
Isolated nuclei that were fixed with osmium tetroxide show an intact nuclear membrane. These nuclei also demonstrate that there are no organelles attached to the membrane and that the nuclear sample is free from contamination.
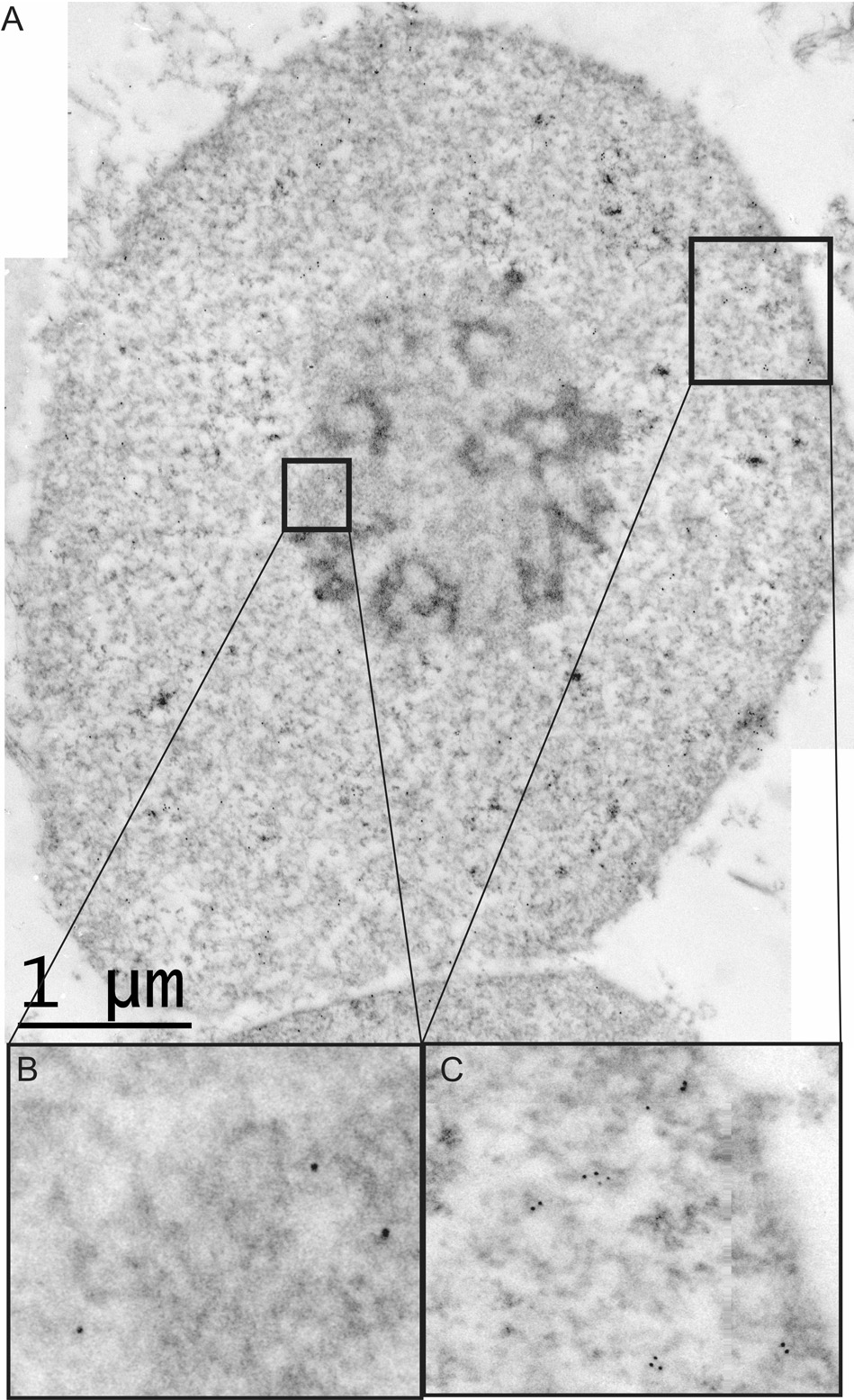
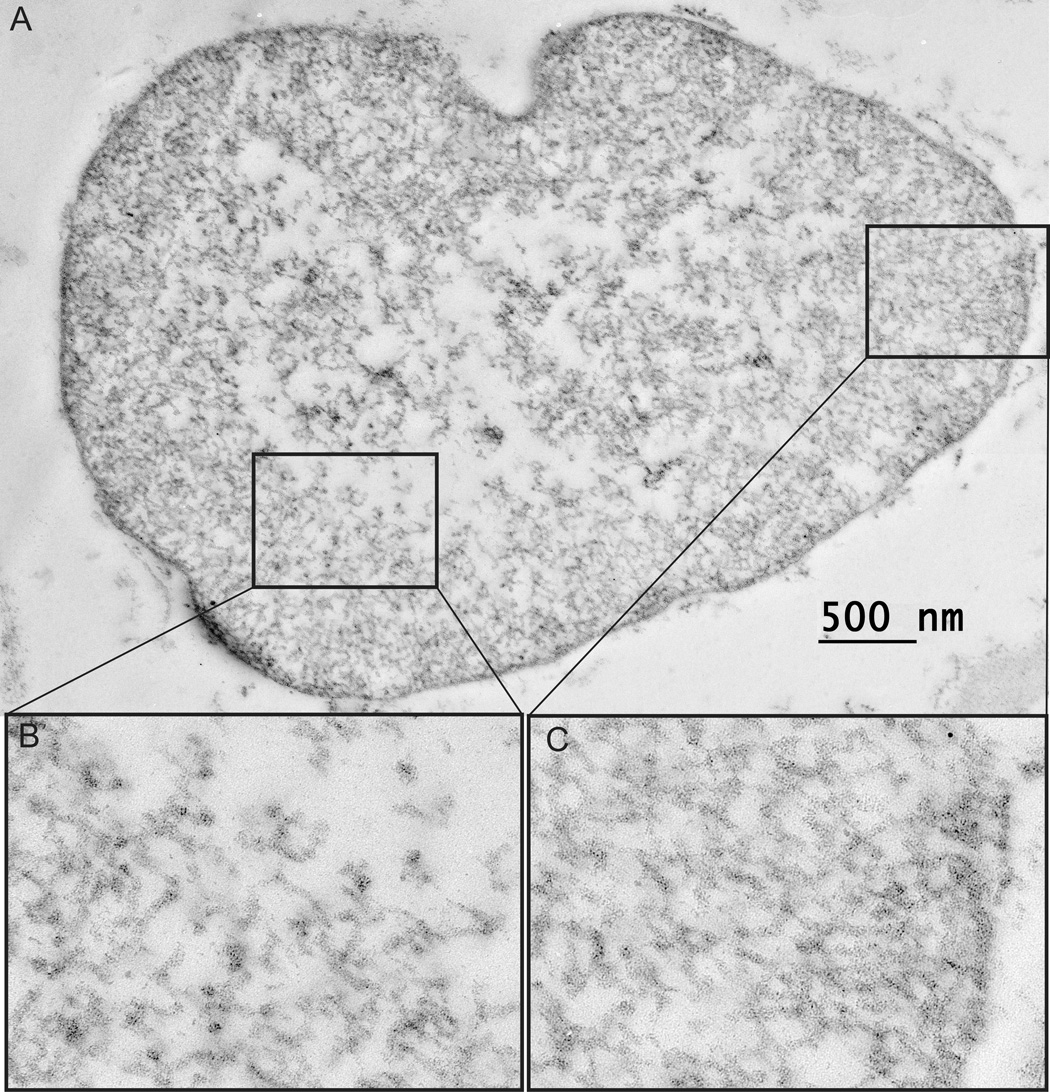
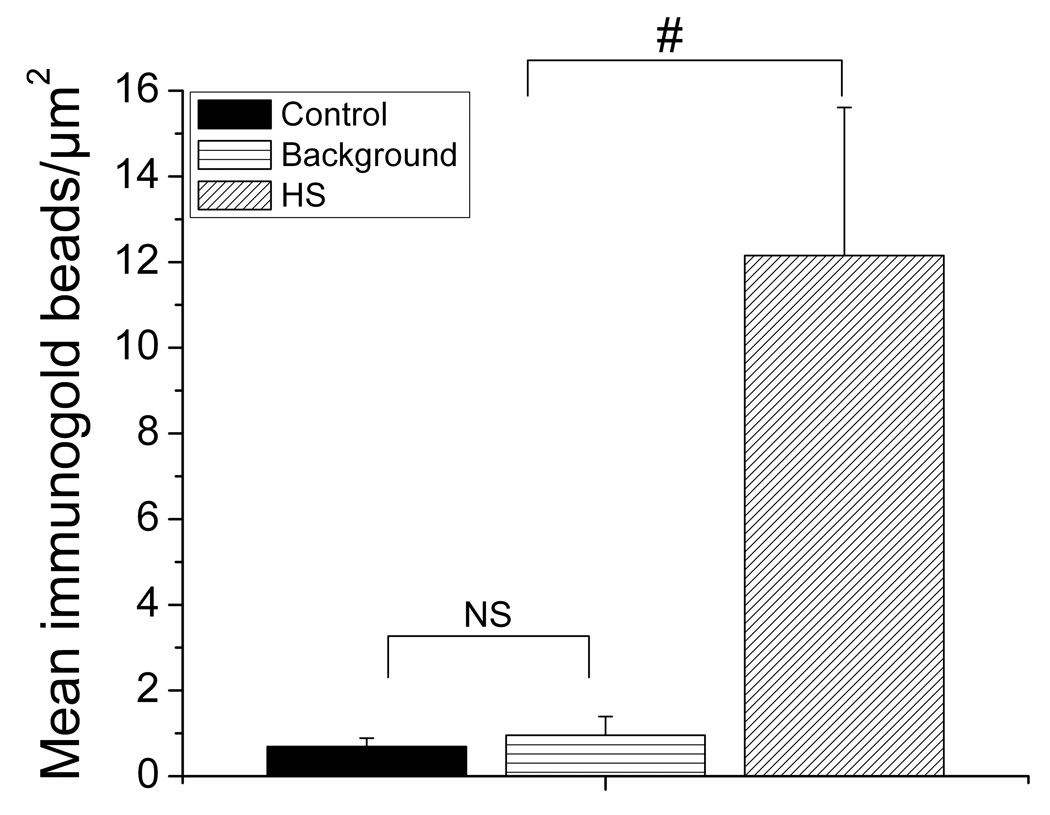
The immunogold-electron micrographs of the isolated nuclei show a robust labeling of NK3R in the nuclei isolated from osmotic-challenged animals as compared to control animals (Figure 4 and Figure 5). The number of immunogold beads in nuclei and background areas were compared in control and animals treated with 2 M NaCl using a 2 way (treatment group, area) ANOVA. In control animals, there was a similar, low number of immunogold beads in nuclei and background areas, p=0.3. The main effect of group and group × area interaction reflected that there were significantly more gold beads in nuclei of animals treated with 2 M NaCl than in control nuclei or in background areas, P’s<0.0001. Quantification (Figure 6) of the immunogold labeling shows that, labeling of the NK3R increases from 0.7 beads/µm2 (SEM ± 0.14) in control animals and to 12.15 beads/µm2 (SEM ± 3.40) following the osmotic challenge. The TEM provides the resolution to determine the sub-nuclear distribution of the NK3R. Immunogold labeling for NK3R was present throughout the nucleus and presented in clusters. This grouping of the receptor in the nucleus is of interest and might suggest aggregations with other nuclear proteins. NK3R immunogold labeling was also present within the nucleolus. This observation requires further study as there was a small number of nucleoli imaged in the nuclear micrographs. Collectively, the micrographs establish that NK3R has been transported to, and internalized within the nucleoplasm.
Figure 4.
(A) An isolated nucleus from that shows immunogold labeling for the NK3R. An electron micrograph of an isolated nucleus from a salt challenged animal. The high density of immunogold labeling within the nucleus demonstrate that the receptor is taken into the nucleus after activation. (C) Shows the clustered labeling of the NK3R within the nucleus. These clusters are found throughout the nucleus. (B) We detect a small amount of labeling within the nucleolus. This labeling suggests the association of the NK3R with sub-nuclear organelles.
Figure 5.
(A) This is an isolated nucleus from a control animal. This nucleus lacks immunogold labeling of the NK3R showing that the receptor is not in the nucleus before activation. (B, C) There is virtually no immunogold labeling of the NK3R within the control nuclei with the exception of one gold bead found by the nuclear membrane in B.
Figure 6.
Quantification of the immunogold bead labeling of the NK3R per µm2 within isolated nuclei from the PVN. Osmotic challenged animals showed a great increase in the number of immunogold beads labeling the NK3R. NS = no significance, # shows significant difference between control and background immunogold labeling (P’s < 0.0001). Error bars indicate SEM.
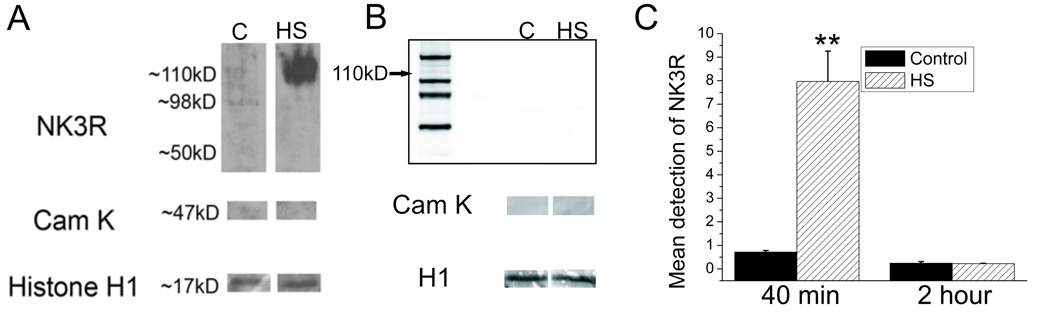
Western blot staining was used to quantify the change in nuclear accumulation of the NK3R protein following the osmotic challenge. Western blot staining of NK3R protein extracted from the isolated nuclei of the PVN was undetectable in control samples. However a robust staining of nuclear NK3R (Figure 7-A) was detected 40 minutes following the acute hyperosmotic challenge. To normalize the protein loads in both control and experimental samples each sample was probed for the presence of the histone H1. The detected levels of NK3R were normalized against the levels of H1 for every sample. This provides a method for the quantification of NK3R across the different experimental states. Quantification of the band intensity from the Western blots demonstrated a 10 fold increase (Figure 7-C) in the labeling density for NK3R 40 minutes after the administration of the osmotic challenge (p< 0.004). This nuclear immunoreactivity returned to undetectable levels 2 hours following the IG hypertonic saline load (Figure 7-B).
Figure 7.
Quantification of NK3R labeling from protein extracted from the isolated nuclei of the PVN. (A) Western blot for NK3R of proteins extracted from isolated nuclei from control and salt challenged animals. Receptor levels are undetectable in the control animals but are easily seen in the salt challenged animals. Samples are also devoid of CaMK1 labeling. (B) Western blot for NK3R from isolated nuclei of an animal sacrificed 2 hours following the hypertonic saline IG load. This demonstrates that the NK3R is only present in the nucleus for a short time following the salt challenge. (C) Quantification of labeling for the NK3R from western blots of control and salt challenged animals at both 40 minutes and 2 hours. (n=4 per group, ** p < 0.004, one-way ANOVA). Error bars indicate SEM.
To confirm that the NK3R expression was due to the receptor being translocated into the nucleus and not from cytoplasmic contamination each sample was stained for CaMK1, and SCAMP5. Nuclear samples were devoid of any CaMK1 and SCAMP5 staining. These controls show that the accumulation of nuclear NK3R protein was due to the active transport of the receptor to the nucleus and not from cytoplasmic contamination of the nuclear isolations.
Discussion
Nuclear Transport
The NK3R is distinct in the tachykinin family of G-protein coupled receptors (GPCRs) in that it is the only one that is reported to contain a nuclear localization sequence (Lee et al., 2004). Also, immunohistochemical results suggest that NK3R was detected in the nucleus of magnocellular neurons after activation by osmotic and hypotensive stimuli (Howe et al., 2004; Haley and Flynn, 2006); no such trafficking has been described for other tachykinin receptors (Mantyh et al., 1995).
As pointed out earlier, immunohistochemical detection of nuclear NK3R is open to alternative interpretations. Here we have confirmed these previous results using specific methods to better characterize and quantify the sub-nuclear localization of the NK3R. One of the issues is the specificity of the antibody. The K7 antibody used in the quantification of the nuclear NK3R is specific for the receptor as determined by the siRNA. The K7 antibody and siRNA target two different regions along the receptor that are both specific to the NK3R. The ability of the siRNA to inhibit the expression of the same protein targeted by the antibody (Figure 1-A) provides strong evidence that the antibody is specific for the NK3R, and that the signal we see in western blots and in the TEM micrographs from the isolated nuclei is, in fact, the NK3R. If the antibody was detecting some other nuclear protein the siRNA would not have been able to interfere with the labeling of that secondary protein. The K7 antibody was also pre-absorbed with the peptide. This is a classical method to demonstrate antibody specificity, but it has its limitations (Swaab et al., 1977; Burry, 2000).
TEM provides the resolution not afforded by other techniques. The nuclear micrographs demonstrate that the isolated nuclei are free from other cytoplasmic contaminants that might result in a false positive signal. Immunogold labeling of the NK3R established that the NK3R is present within the nucleoplasm and not just associating with the external surface of the nuclear membrane. The movement of proteins into, and throughout the nucleus is an important factor in regulating gene expression and RNA splicing (Phair and Misteli, 2000; Sacco-Bubulya and Spector, 2002). As stated earlier the receptor immunoreactivity tended to cluster together in the nucleoplasm. The grouped labeling of the NK3R within the nucleus suggests the association of the receptor with other intranuclear proteins and organelles. The punctate, grouped pattern of NK3R labeling has a similar appearance to that of the nuclear paraspeckles (Fox et al., 2002). These paraspeckle proteins have been identified within various regions of the nucleus, including the nucleolus of cells (Fox et al., 2002). This adds to the idea of a possible interaction of NK3R with paraspeckle proteins because the NK3R was also detected within the nucleolus of these neurons. Interestingly, several studies show that osmotic challenge dramatically affects the morphology of the nucleolus (Armstrong et al., 1977) and that this change is related to ribosome biogenesis to support the synthesis of new hormone peptides (Berciano et al., 2002). The association of the NK3R with these nuclear organelles strengthens the hypothesis that the receptor is playing an active role in gene expression within the nucleus of neurons after it has been activated and internalized.
Western data show a large change in the accumulation of NK3R within the isolated nuclei following the osmotic challenge. This is consistent with previous confocal microscopy data that showed no nuclear NK3R under basal conditions and significant nuclear labeling under the challenged conditions (Howe et al., 2004; Haley and Flynn, 2006) and also with the present immunogold electron microscopy data. The westerns also demonstrate that this accumulation of NK3R immunoreactivity within the nuclei is time dependent as the immunoreactivity for NK3R returns to control levels 2 hours following the hypertonic saline load. This is consistent with other reports on the nuclear export of receptors (Savory et al., 1999). The lack of labeling for SCAMP5 and CaMK1 confirm that the nuclei isolated are free from any cytoplasmic contamination and that the detected NK3R is from within the nuclei. The data presented show that the NK3R is a dynamic receptor that upon activation by an osmotic stimulus in-vivo, is transported to the nucleus of magnocellular neurons in the PVN. To our knowledge this is the only study using techniques that provide the resolution to quantify the accumulation of the NK3R in the nucleoplasm in an in-vivo model following an osmotic challenge. The significance of this translocation to the nucleus is still being investigated, but it opens many avenues for how these magnocellular neurons respond to receptor activation and as to what role the receptor plays in the intracellular signaling cascade that it activates.
As stated above the NK3R has a putative NLS (amino acids 348–351) on the cytoplasmic tail of the receptor (Lee et al., 2004). Through this NLS the NK3R can associate with importins and then be actively transported from the cytoplasm into the nucleus through the nuclear pore complex. This is a known mechanism for energy dependent transport into the nucleus (Moroianu, 1999; Johnson et al., 2004). It is suggested that the association of the receptor with the importins is the rate limiting step in the nuclear transport process (Jans et al., 2000) and that there needs to be a sufficient concentration of the receptor available inside the cell to bind with the importins. It is also suggested that the types of importins present in the cell and their affinity for the NLS present on the target molecule can greatly affect the rate of transport into the nucleus (Nigg, 1997; Jans et al., 2000). Earlier reports have shown high levels of membrane bound NK3R with corresponding low levels of internalized NK3R in the cytoplasm before receptor activation (Haley and Flynn, 2006). It is only after activation that the receptor is internalized and we see the nuclear presence of the receptor. This nuclear transport may be due to the increased availability of the receptor within the cell. As the receptor is internalized in greater numbers it is then more likely to associate with the importins and be taken into the nucleus. Also, the activation and internalization of the receptor may be a necessary step in unmasking the NLS so that it can associate with importins and be taken into the nucleus (Henkel et al., 1992; Nigg, 1997; Briggs et al., 1998; Jans et al., 2000). Taken together these data suggest that the receptor that is present in the nucleus after activation is coming from the membrane and not from an intracellular pool of NK3R.
The nuclear targeting of the NK3R may alter the gene expression of these magnocellular neurons and thereby change the manner in which these neurons respond to osmotic stimuli. Hyperosmotic stimuli alters levels of mRNA, and protein in the magnocellular neurons (Meister et al., 1990). Microarray studies have shown that RNA expression in the SON changes because of dehydration (Ghorbel et al., 2006). Acute osmotic challenges have been shown to increase the expression of many immediate early genes like c-fos, junB, NGFI-A, and NGFI-B in both the PVN and the SON (Kawasaki et al., 2005). It has also been shown that blocking the activation and trafficking of the NK3R with an antagonist, prevents the up-regulation of c-Fos in these magnocellular neurons (Haley, unpublished observations). In situ hybridization studies of the SON demonstrate that different genes are up-regulated in animals in chronic hyperosmotic versus hypoosmotic states (Glasgow et al., 2000). While this study was done in the SON, both the SON and PVN express the NK3R and both are important in the release of OT and VP. In both nuclei, nuclear translocation of the NK3R has been reported (Howe et al., 2004; Haley and Flynn, 2006). Here we propose that the nuclear transport of the NK3R after activation is an important step in the signaling cascade of the receptor and in the regulation of genes in these magnocellular neurons. The transport of the activated membrane receptor to the nucleus provides a second level of control to the receptor’s activation and helps to refine the signal and the response at the level of the nucleus (Jans and Hassan, 1998; Subramaniam et al., 2001; Johnson et al., 2004) Nuclear transport of membrane receptors has been shown to play a significant role in cell signaling at the nuclear level (Jans and Hassan, 1998; Bkaily et al., 2003). Based on this evidence it is reasonable to hypothesize that the NK3R also has an important role in cell signaling at the nuclear level.
Identifying the role that NK3R has upon entering the nucleus is an important step in understanding the response of magnocellular neurons after an osmotic challenge. NK3R signaling is essential for the systemic release of VP and OT in response to hyperosmolarity and hypotension (Haley and Flynn, 2007) and it is very likely that NK3R plays a role in gene expression and long term modifications of the cellular response. This seemingly important role of NK3R in the cell nucleus may have larger significance than osmoregulation within the brain. The NK3R is not only expressed in the PVN and SON, but is widely expressed throughout the brain. Furthermore, a number of neurological disorders like epilepsy, long term hyperalgesia following peripheral inflammation (Ackley et al., 2001), anxiety, and depression (Panocka et al., 2001; Salome et al., 2006), have all been linked to the dysregulation of NK3R. Studies that seek to understand the role of NK3R within the nucleus may help in our understanding of the role that NK3R has in these neurological disorders. These studies will also serve as a model for the in-vivo study of the nuclear transport and the genomic regulation of other GPCRs, such as angiotensin (Yang et al., 1997; Bkaily et al., 2003; Morinelli et al., 2007) and bradykinin (Lee et al., 2004) which have been studied in cell cultures.
Acknowledgements
We would like to thank Kevin G. Dunbar and Steven W. Flynn for their help in quantifying immunogold labeling and micrograph preparation.
This work was supported by NIH grants RR15640, DK50586, and NS57823 awarded to Dr. F.W.F.
Abbreviations
- AT1AR
Angiotensin 1A Receptor
- CaMK1
Calcium Calmodulin Kinase 1
- GPCRs
G-protein Coupled Receptors
- G-proteins
GTP Binding Proteins
- H1
Histone H1
- NK3R
Neurokinin 3 Receptor
- NKB
Neurokinin B
- NLS
Nuclear Localization Sequence
- OT
Oxytocin
- PBS
Phosphate Buffers Saline
- PVN
Paraventricular Nucleus
- SCAMP5
Secretory Carrier Associated Membrane Protein 5
- siRNA
Small Interfering RNA
- SON
Supraoptic Nucleus
- TEM
Transmission Electron Microscope
- VP
Vasopressin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ackley MA, Asghar AUR, Worsley MA, King AE. Peripheral inflammation reduces the response of spinal dorsal horn neurons to an NK3 receptor agonist. Neuroscience Letters. 2001;308:13–16. doi: 10.1016/s0304-3940(01)01968-1. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Gregory WA, Hatton GI. Nucleolar proliferation and cell size changes in rat supraoptic neurons following osmotic and volemic challenges. Brain Res Bull. 1977;2:7–14. doi: 10.1016/0361-9230(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Bealer SL, Flynn FW. Central neurokinin 3 receptors increase systemic oxytocin release: interaction with norepinephrine. Exp Neurol. 2003;184:1027–1033. doi: 10.1016/j.expneurol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Berciano MT, Villagra NT, Pena E, Navascues J, Casafont I, Lafarga M. Structural and functional compartmentalization of the cell nucleus in supraoptic neurons. Microsc Res Tech. 2002;56:132–142. doi: 10.1002/jemt.10013. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F, Jr, Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol. 2003;81:274–287. doi: 10.1139/y03-007. [DOI] [PubMed] [Google Scholar]
- Briggs LJ, Stein D, Goltz J, Corrigan VC, Efthymiadis A, Hubner S, Jans DA. The cAMP-dependent Protein Kinase Site (Ser312) Enhances Dorsal Nuclear Import through Facilitating Nuclear Localization Sequence/Importin Interaction. J Biol Chem. 1998;273:22745–22752. doi: 10.1074/jbc.273.35.22745. [DOI] [PubMed] [Google Scholar]
- Buell G, Schulz MF, Arkinstall SJ, Maury K, Missotten M, Adami N, Talabot F, Kawashima E. Molecular characterisation, expression and localisation of human neurokinin-3 receptor. FEBS Lett. 1992;299:90–95. doi: 10.1016/0014-5793(92)80107-r. [DOI] [PubMed] [Google Scholar]
- Burry RW. Specificity Controls for Immunocytochemical Methods. J Histochem Cytochem. 2000;48:163–166. doi: 10.1177/002215540004800201. [DOI] [PubMed] [Google Scholar]
- Chawala M, Gutierrez G, Young W, III, McMullen N, Rance N. Localization of neurons expressing Substance P and Neurokinin B gene transcripts in the human hypothalamus and basal forebrain. The Journal of Comparative Neurology. 1997;384:429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Colin I, Blondeau C, Baude A. Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors. Neuroscience. 2002;115:1023–1033. doi: 10.1016/s0306-4522(02)00541-9. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ding Y-Q, Lu B-Z, Guan Z-L, Wang D-S, Xu J-Q, Li J-H. Neurokinin B receptor (NK3)-containing neurons in the paraventricular and supraoptic nuclei of the rat hypothalamus synthesize vasopressin and express Fos following intravenous injection of hypertonic saline. Neuroscience. 1999;91:1077–1085. doi: 10.1016/s0306-4522(98)00643-5. [DOI] [PubMed] [Google Scholar]
- Ding Y, Lawrence CE. Rational design of siRNAs with the Sfold software. In: Appasani Krishnarao., editor. RNA Inteference Technology. New York: Cambridge University Press; 2005. pp. 129–138. [Google Scholar]
- Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI. Paraspeckles: a novel nuclear domain. Curr Biol. 2002;12:13–25. doi: 10.1016/s0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- Ghorbel MT, Sharman G, Hindmarch C, Becker KG, Barrett T, Murphy D. Microarray screening of suppression subtractive hybridization-PCR cDNA libraries identifies novel RNAs regulated by dehydration in the rat supraoptic nucleus. Physiol Genomics. 2006;24:163–172. doi: 10.1152/physiolgenomics.00229.2005. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Murase T, Zhang B, Verbalis JG, Gainer H. Gene expression in the rat supraoptic nucleus induced by chronic hyperosmolality versus hyposmolality. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1239–R1250. doi: 10.1152/ajpregu.2000.279.4.R1239. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol. 2007;293:R931–R937. doi: 10.1152/ajpregu.00196.2007. [DOI] [PubMed] [Google Scholar]
- Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1242–R1250. doi: 10.1152/ajpregu.00773.2005. [DOI] [PubMed] [Google Scholar]
- Hamada M, Ohtsuka T, Kawaida R, Koizumi M, Morita K, Furukawa H, Imanishi T, Miyagishi M, Taira K. Effects on RNA interference in gene expression (RNAi) in cultured mammalian cells of mismatches and the introduction of chemical modifications at the 3'-ends of siRNAs. Antisense Nucleic Acid Drug Dev. 2002;12:301–309. doi: 10.1089/108729002761381285. [DOI] [PubMed] [Google Scholar]
- Henkel T, Zabel U, van Zee K, Muller JM, Fanning E, Baeuerle PA. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-[kappa]B subunit. Cell. 1992;68:1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Howe HE, Somponpun SJ, Sladek CD. Role of Neurokinin 3 Receptors in Supraoptic Vasopressin and Oxytocin Neurons. J Neurosci. 2004;24:10103–10110. doi: 10.1523/JNEUROSCI.3164-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard C, Singleton D, Rauch M, Jayasinghe S, Cafiso D, Castle D. The secretory carrier membrane protein family: structure and membrane topology. Mol Biol Cell. 2000;11:2933–2947. doi: 10.1091/mbc.11.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, Khvorova A, Linsley PS. Position-specific chemical modification of siRNAs reduces "off-target" transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans DA, Hassan G. Nuclear targeting by growth factors, cytokines, and their receptors: a role in signaling? Bioessays. 1998;20:400–411. doi: 10.1002/(SICI)1521-1878(199805)20:5<400::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. Bioessays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Yamaguchi K, Saito J, Ozaki Y, Mera T, Hashimoto H, Fujihara H, Okimoto N, Ohnishi H, Nakamura T, Ueta Y. Expression of immediate early genes and vasopressin heteronuclear RNA in the paraventricular and supraoptic nuclei of rats after acute osmotic stimulus. J Neuroendocrinol. 2005;17:227–237. doi: 10.1111/j.1365-2826.2005.01297.x. [DOI] [PubMed] [Google Scholar]
- Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O'Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Lessard A, Laurin M, Yamaguchi N, Couture R. Central anti-hypertensive effect of tachykinin NK3 receptor antagonists in rat. Eur J Pharmacol. 2004;486:75–83. doi: 10.1016/j.ejphar.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B, Cortes R, Villar MJ, Schalling M, Hokfelt T. Peptides and transmitter enzymes in hypothalamic magnocellular neurons after administration of hyperosmotic stimuli: comparison between messenger RNA and peptide/protein levels. Cell Tissue Res. 1990;260:279–297. doi: 10.1007/BF00318631. [DOI] [PubMed] [Google Scholar]
- Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89:1269–1290. doi: 10.1016/s0306-4522(98)00349-2. [DOI] [PubMed] [Google Scholar]
- Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L, Ullian ME. Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol Cell Physiol. 2007;292:C1398–C1408. doi: 10.1152/ajpcell.00337.2006. [DOI] [PubMed] [Google Scholar]
- Moroianu J. Nuclear import and export pathways. J Cell Biochem. 1999 Suppl 32–33:76–83. doi: 10.1002/(sici)1097-4644(1999)75:32+<76::aid-jcb10>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- Naruse M, Naruse K, Mckenzie JC, Schelling P, Inagami T. Regional Distribution of Renin and Angiotensinogen in the Brain of Normotensive (Wky) and Spontaneously Hypertensive (Shr) Rats. Brain Research. 1985;333:147–150. doi: 10.1016/0006-8993(85)90135-0. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Current Biology. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- Panocka I, Massi M, Lapo I, Swiderski T, Kowalczyk M, Sadowski B. Antidepressant-type effect of the NK3 tachykinin receptor agonist aminosenktide in mouse lines differing in endogenous opioid system activity. Peptides. 2001;22:1037–1042. doi: 10.1016/s0196-9781(01)00438-7. [DOI] [PubMed] [Google Scholar]
- Phair RD, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 Parathyroid Hormone Receptor (PTH1R) Nuclear Trafficking: Regulation of PTH1R Nuclear-Cytoplasmic Shuttling by Importin-{alpha}/{beta} and Chromosomal Region Maintenance 1/Exportin 1. Endocrinology. 2007;148:2282–2289. doi: 10.1210/en.2007-0157. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Israel A, Plunkett LM, Kurihara M, Shigematsu K, Correa FM. Quantitative distribution of angiotensin II binding sites in rat brain by autoradiography. Peptides. 1986;7:679–687. doi: 10.1016/0196-9781(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Sacco-Bubulya P, Spector DL. Disassembly of interchromatin granule clusters alters the coordination of transcription and pre-mRNA splicing. J Cell Biol. 2002;156:425–436. doi: 10.1083/jcb.200107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Selective blockade of NK2 or NK3 receptors produces anxiolytic- and antidepressant-like effects in gerbils. Pharmacology Biochemistry and Behavior. 2006;83:533–539. doi: 10.1016/j.pbb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin F, Roosterman D, Bunnett NW. The third intracellular loop and carboxyl tail of neurokinin 1 and 3 receptors determine interactions with {beta}-arrestins. Am J Physiol Cell Physiol. 2003;285:C945–C958. doi: 10.1152/ajpcell.00541.2002. [DOI] [PubMed] [Google Scholar]
- Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Burchard J, Linsley PS, Aronin N, Xu Z, Zamore PD. Designing siRNA That Distinguish between Genes That Differ by a Single Nucleotide. PLoS Genet. 2006;2 doi: 10.1371/journal.pgen.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK(3) receptor agonist senktide. J Comp Neurol. 2000;426:413–428. doi: 10.1002/1096-9861(20001023)426:3<413::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Subramaniam PS, Torres BA, Johnson HM. So many ligands, so few transcription factors: a new paradigm for signaling through the STAT transcription factors. Cytokine. 2001;15:175–187. doi: 10.1006/cyto.2001.0905. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Pool CW, Van Leeuwen FW. Can specificity ever be proved in immunocytochemical staining [letter] J Histochem Cytochem. 1977;25:388–391. doi: 10.1177/25.5.325124. [DOI] [PubMed] [Google Scholar]
- Thevenin D, Lazarova T, Roberts MF, Robinson CR. Oligomerization of the fifth transmembrane domain from the adenosine A2A receptor. Protein Sci. 2005;14:2177–2186. doi: 10.1110/ps.051409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu D, Vinson GP, Raizada MK. Involvement of MAP Kinase in Angiotensin II-Induced Phosphorylation and Intracellular Targeting of Neuronal AT1 Receptors. J Neurosci. 1997;17:1660–1669. doi: 10.1523/JNEUROSCI.17-05-01660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]