Abstract
Nitric oxide (NO) has been implicated in pain processing at the spinal level, but the mechanisms mediating its effects remain unclear. In the present work, we studied the organization of the major downstream effector of NO, soluble guanylyl cyclase (sGC), in the superficial dorsal horn of rat. Almost all neurokinin 1 (NK1) receptor-positive neurons in lamina I (a major source of ascending projections) were strongly immunopositive for sGC. Many local circuit neurons in laminae I-II also stained for sGC, but less intensely. Numerous fibers, presumably of unmyelinated primary afferent (C fiber) origin, stained for calcitonin gene-related peptide or isolectin B4, but none of these was immunopositive for sGC. These data, along with immunoelectron microscopy results, imply that unmyelinated primary afferent fibers terminating in the superficial dorsal horn lack sGC. Double labeling showed that neuronal nitric oxide synthase (nNOS) seldom colocalized with sGC, but nNOS-positive structures were frequently closely apposed to sGC-positive structures, suggesting that in the superficial dorsal horn NO acts mainly in a paracrine manner. Our data suggest that the NK1 receptor-positive projection neurons in lamina I are a major target of NO released in superficial dorsal horn. NO may also influence local circuit neurons, but it does not act on unmyelinated primary afferent terminals via sGC.
Keywords: cGMP, C fibers, neurokinin 1 receptor, nitric oxide, pain, substantia gelatinosa
Nitric oxide (NO), a freely-diffusible gas, has been implicated in pain processing in the spinal cord. Its synthetic enzyme, neuronal nitric oxide synthase (nNOS), is expressed at high levels in superficial dorsal horn (Dun et al., 1992; Valtschanoff et al., 1992; Saito et al., 1994). Levels of nNOS and NADPH diaphorase (a histochemical marker for NOS) increase in the dorsal horn in response to injury or inflammation (Maihofner et al., 2000; Wu et al., 2001; Liang and Clark, 2004). Importantly, intrathecal administration of NOS inhibitors attenuates the pain response (Malmberg and Yaksh, 1993; Osborne and Coderre, 1999), while administration of NO donors can induce hyperalgesia (Kitto et al., 1992; Inoue et al., 1997). It is thus thought that NO may modulate synaptic efficacy in the superficial dorsal horn, though the exact mechanisms are still unclear (Willis, 2002). This concept has led to the suggestion that the NO signaling pathway may provide a novel target for therapeutic interventions in the management of pain (Luo and Cizkova, 2000; Thomsen and Olesen, 2001).
In the superficial dorsal horn, NOS concentrates in a subset of islet cells lying at the ventral border of laminae II, which send processes that make plexi in lamina I and IIi (Valtschanoff et al., 1992). nNOS is a calcium-dependent enzyme; indirect evidence suggests that glutamate released from primary afferents may allow calcium entry through N-methyl-D-aspartate channels, leading to release of NO from these cells (Li et al., 1994; Morris et al., 1994; Aimar et al., 1998; Kawamata and Omote, 1999). Newly-synthesized NO can then diffuse to act on neighboring targets within the superficial dorsal horn, possibly including projection neurons, local circuit interneurons and/or primary afferent terminals (Millan, 1999). However, despite numerous in vitro studies (see, for example, Garry et al., 1994; Lin et al., 1999; Wu et al., 2001), the targets of NO in the superficial dorsal horn in vivo are still unclear.
The effects of NO are mediated mainly via soluble guanylyl cyclase (sGC), which catalyzes the formation of the intracellular second messenger cyclic guanosine monophosphate (cGMP) upon activation by NO (Schmidt et al., 1993; Bellamy and Garthwaite, 2002). The NO-sGC-cGMP pathway may help to mediate long-term potentiation in the forebrain (Schuman and Madison, 1994; Garthwaite and Boulton, 1995; Hawkins et al., 1998). Pharmacological and electrophysiological evidence from the superficial dorsal horn implicates cGMP (presumably synthesized by sGC) in pain (Morris et al., 1994; Lin et al., 1997; Kawamata and Omote, 1999). However, while a previous immunohistochemical study reported sGC surrounding the central canal, little is known about sGC in the dorsal horn (Maihofner et al., 2000), though this information is crucial for understanding the role of the NO-cGMP pathway in pain processing.
Functional sGC is a heterodimer comprising one α and one β subunit (Kamisaki et al., 1986; Gibb et al., 2003). Four subunits (α1, α2, β1 and β2) have been described, but only α1β1 and α2β1 heterodimers have been found in the CNS (Koesling et al., 2004). In the present work, we investigated the cellular and subcellular distribution of sGC in the dorsal horn using immunohistochemistry for the β1 subunit, previously shown to be an effective marker for sGC enzyme (Ding et al., 2004). We combined immunohistochemistry for sGC with that for nNOS and for other neuronal markers to determine whether NO's principal target is primary afferent fibers, local circuit neurons, or ascending projection neurons; and whether it acts predominantly in an autocrine or paracrine manner.
MATERIALS AND METHODS
Animal and tissue preparation
Care and treatment of animals were strictly in accordance with institutional and NIH guidelines. Eight adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were anesthetized deeply with sodium pentobarbital (60 mg/kg, i.p.), then perfused transcardially with saline (0.9% NaCl) followed by fixative. For light microscopy (LM), fixation was with freshly depolymerized 4% paraformaldehyde (PF) in 0.1 M sodium phosphate buffer (PB), pH 7.4; for electron microscopy (EM) or GABA immunostaining, glutaraldehyde (0.1 %) was added into the above fixative. Spinal cords were removed and postfixed in 4% PF for 2 hours. Tissue blocks containing lumbar segments 4 (L4) and L5 were mounted on a Vibratome. Transverse and parasagittal sections were cut at 50 μm. For Western blot analysis, spinal cord tissues containing lumbar enlargement was quickly removed from two deeply-anesthetized rats and frozen in liquid nitrogen. Tissues were stored at −80°C until further processed.
Primary antibodies
The anti-sGCβ1 antibody (#160897; Cayman Chemicals, Ann Arbor, MI) was raised against amino acids 188−207 of the β1 subunit of sGC. Its specificity has been documented in previous studies by Western blot analysis and by colocalization with another antibody against a different part of sGCβ1 in rat brain (Ding et al., 2004; 2005). For detailed information and controls regarding the primary antibodies used in this study, see Table 1.
Table 1.
Primary antibodies used in this study.
| Antibody | Dilution | Description | Characterization of specificity | Controls |
|---|---|---|---|---|
| sGCβ1 | 0.5 μg/ml; 0.05 μg/ml (for TSA) | Rabbit antibody (Cayman Chemicals, Ann Arbor, MI; #160897) prepared against a synthetic peptide representing amino acids 188−207 from the β1 subunit of rat sGC. | Recognizes a single band at ∼70 kDa on Western blot of rat brain Ding et al., 2004) and spinal cord (Fig. 1). | Staining with this antibody colocalized in rat brain with another antibody against a different part (amino acids 593−614) of sGCβ1 (Ding et al., 2005). |
| NK1 receptor | 1: 1,000 | Rabbit antibody (Advanced Targeting Systems, San Diego, CA; AB-N04) prepared against a peptide corresponding to amino acids 393−407 of the rat NK1 receptor. | Recognizes a protein band at 80−90 kDa on Western blots of membranes prepared from cells transfected with the rat substance P receptor (Vigna et al., 1994). | Staining in rat spinal cord was blocked by preabsorbing the antiserum with the immunizing peptide (Mantyh et al., 1995) |
| GABA | 0.1 μg/ml | Rabbit antibody (Sigma, St Louis, MO; A2052) raised against a GABA-BSA conjugate. | Reacts with GABA and GABA-KLH, but not with BSA in dot blot immunoassay manufacturer's product information). | Preabsorption of the antiserum with free GABA blocked immunostaining in rat dorsal horn (Bernardi et al., 1995) |
| GFAP | 1: 1,000 | Mouse monoclonal antibody (Sigma; clone G-A-5) prepared against purified GFAP from pig spinal cord. | Recognizes a single band of 51 kDa on Western blots of glioma cell extract (Debus et al., 1983). | Immunostaining was not detected in cultured brain cell from GFAP knockout mice (Pekny et al., 1998). |
| CGRP | 1: 5,000 | Guinea pig antibody (Peninsula, San Carlos, CA; T-5027) prepared against human α-CGRP conjugated to BSA or thyroglobin. | Specificity was verified with radioimmunoassay by the manufacturer. | The staining pattern of this antibody in rat dorsal horn is identical to previous descriptions (Chung et al., 1988). |
| Synaptophysin | 1: 1,000 | Mouse monoclonal antibody (Sigma; clone SVP-38). A synaptosome preparation from rat retina was used as the immunogen. | Recognizes one band of ∼38 kDa on immunoblot of synaptosomal fraction from rat cerebral cortex (Devoto and Barnstable, 1987). | Immunostaining was not detected in brain tissue from synaptophysin-deficient mice (Eshkind and Leube, 1995) |
| nNOS | 0.25 μg/ml | Rabbit antibody (Zymed, South San Francisco, CA; 61−7000) prepared against a recombinant protein consisting of 195 amino acids from the N-terminal of rat nNOS protein. | Recognizes a band at ∼150 kDa on Western blot of rat brain tissue (Burette et al., 2002). | Immunostaining was not detected in brain tissue from nNOS knockout mice (Burette et al., 2002). |
Western blot analysis
Lumbar spinal cord was homogenized in ice-cold lysis buffer (50 mM Tris base, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Nonidet P40, 0.1% SDS, 2 mM EDTA), containing protease inhibitors (1 mM each of pepstatin, leupeptin, aprotinin, and phenylmethylsulfonyl fluoride; all from Sigma), then centrifuged at 16,000 g at 4 °C for 20 min. The supernatant was collected and protein content determined using a BCA kit (Pierce Biotechnology Inc., Rockford, IL). Proteins were separated by SDS-PAGE (10%), transferred to PVDF membranes (BioTrace; Pall Corporation, Ann Arbor, MI), and blocked with 5% fat-free dry milk (Carnation) in a mixture of 20 mM Tris, 137 mM NaCl, pH 7.6, and 0.1% Tween-20. Anti-sGCβ1 antibody was added to the blocking solution (see above) at 0.5 μg/ml. Immune complexes were visualized using an ECL immunodetection kit; bands were visualized with a Kodak imaging station.
Immunohistochemistry
Sections were chosen for immunohistochemistry with reference to structures revealed on Nissl-stained sections. For immunoperoxidase, free-floating sections were treated for 30 minutes with 3% H2O2 in phosphate-buffered saline (PBS) to quench endogenous peroxidase, preincubated in 10% normal donkey serum (NDS) for 30 minutes, and incubated overnight on a shaker at room temperature with sGCβ1 primary antibody at 0.5 μg/ml. Sections were then incubated for 3 hours in biotinylated donkey anti-rabbit IgG (6 μg/ml; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and for 1 hour in ExtrAvidin-peroxidase complex (0.5 μg/ml; Sigma, St. Louis, MO); peroxidase was visualized histochemically with diaminobenzidine (DAB). Processed sections were mounted on slides and air dried. Sections were dehydrated with graded alcohol, cleared with xylene, and coverslipped with DPX mountant (BDH Chemicals, Poole, England). To control for method specificity, some sections were processed as above, except that primary or secondary antibodies were omitted. In all such cases, staining was not detected.
For immunofluorescent multiple-labeling of sGC with other antigens, sGCβ1 was first stained using tyramide signal amplification (TSA, Hunyady et al., 1996; Shindler and Roth, 1996). Briefly, formaldehyde-fixed free-floating sections were treated for 30 minutes with 3% H2O2 in PBS and preincubated in 10% NDS for 30 minutes. Sections were incubated overnight on a shaker at room temperature with anti-sGCβ1 primary antibody at 0.05μg/ml, a concentration too low to be detected with conventional fluorophore-conjungated secondary antibody. Sections were then incubated for 3 hours in biotinylated donkey anti-rabbit IgG (6 μg/ml; Jackson). The immunostaining was then revealed with a TSA kit (Renaissance TSA direct kit; DuPont NEN, Wilmington, DE) according to the manufacturer's protocol. Sections were then incubated in the second (and third) primary antibody [anti-neurokinin 1 (NK1) receptor, anti-glial fibrillary acidic protein (GFAP), anti-calcitonin gene-related peptide (CGRP) or anti-synaptophysin] overnight (see Table1 for details). Immunostaining was revealed by incubation for 3 hours with fluorophore-conjugated secondary antibodies (Jackson). For double staining with GABA, we used tissue fixed with a mixture of 4% PF and 0.1% glutaraldehyde. Sections were incubated with 1% sodium borohydride for 30 minutes prior to all steps described above. For double labeling with Griffonia simplicifolia isolectin B4 (IB4), after sGC immunostaining, sections were incubated with FITC-conjugated IB4 (0.2 μg/ml; L2895; Sigma) for 3 hours. Following histological processing, sections were mounted on slides, air-dried and coverslipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA). We performed negative controls by omitting either the first or second primary antibodies. In each case the immunostaining disappeared in the corresponding channel.
For preembedding EM immunohistochemistry, sections were pretreated with 1% sodium borohydride for 30 minutes to quench free aldehyde groups, and further processed as for DAB immunoperoxidase staining for LM. Immunoreacted tissue was rinsed in PB, post-fixed 1 hour in 1% osmium tetroxide, rinsed in PB, rinsed in 0.1 M maleate buffer (MB, pH 6), stained en bloc 1 hour in 1% uranyl acetate in MB, rinsed, then dehydrated in a graded ethanol series followed by propylene oxide, infiltrated with a mixture of Epon and Spurr resins, embedded between two sheets of ACLAR plastic supported by glass slides, and polymerized at 60°C for 36−48 hours. Thin sections mounted on copper grids were counterstained with 5% uranyl acetate and Sato's lead and examined on a Philips Tecnai 12 EM (FEI, Hillsboro, OR).
Image acquisition and processing
Immunoperoxidase-reacted sections were examined with a Leitz DMR microscope (Wetzlar, Germany) under brightfield illumination. Images were acquired with a 12-bit cooled, charge-coupled device camera (Retiga EX, QImaging, Burnaby, BC, Canada) coupled to a Macintosh computer. Openlab software (Improvision Inc, Lexington, MA) was used for image acquisition. Multiple-labeled immunofluorescent images were collected using a Leica TCS SP2 laser scanning confocal microscope (Wetzlar, Germany). Adobe Photoshop (version 8.0; Adobe Systems Inc., San Jose, CA) was used to examine the spatial relationship of the sGCβ1-and nNOS- immunostained puncta by switching between channels. Adobe Photoshop and Corel Draw (version 12; Corel, Ontario, Canada) were used to sharpen images, adjust brightness and contrast, and compose final plates.
RESULTS
Detection of sGCβ1 protein in lumbar spinal cord
We used Western blot analysis to verify the presence of sGCβ in spinal cord. The anti-sGCβ1 antibody detected a single strong band in blots from homogenates of lumbar cord, migrating at ∼70 kDa, corresponding to the predicted size of the β1 subunit of sGC (Fig. 1). These data also confirmed that this antibody is specific in spinal cord tissue.
Fig. 1.
Western blot analysis of sGCβ1 subunit in rat lumbar spinal cord. The anti-sGCβ1 antibody detected a single band at ∼70 kDa, corresponding to β1 subunit.
Immunoperoxidase staining of sGCβ1 in superficial dorsal horn
We performed immunohistochemistry for sGCβ1 on sections of L4 and L5 spinal cord (Fig. 2). sGC immunostaining was widely distributed throughout the gray matter of the superficial dorsal horn. The staining pattern showed a laminar organization: Somatic staining was strongest in lamina I, moderate in lamina IIo, and weak in IIi; likewise, neuropil staining was strong in lamina I, and moderate in lamina II (Fig. 2A). Somatodendritic staining was well-defined in deeper laminae of the dorsal horn. At high magnification, scattered immunopositive somata could be seen in lamina I (Fig. 2B, C). Numerous large puncta (arrows in Fig. 2B) and some processes running parallel to the surface of the gray matter (arrowheads) were densely stained in lamina I. In lamina II, weakly sGCβ1-positive somata lay among a dense plexus of stained processes (Fig. 2B). On parasagittal sections, most stained neurons were elongated along the rostro-caudal axis (Fig. 2C). Immunopositive processes also ran rostro-caudally in laminae I and IIo, but exhibited less orientation in deeper laminae. To examine the subcellular expression of sGC, we performed immunoelectron microscopy. Immunoperoxidase reaction product was mainly found in somata and dendrites (Fig. 2D, E). A few axon terminals were also immunopositive (Fig. 2F); these terminals did not contain dense core vesicles.
Fig. 2.
sGCβ1 immunoperoxidase staining in rat superficial dorsal horn at LM (A-C) and EM (D-F). A, low magnification photomicrograph of immunostaining on transverse section. Staining is seen throughout the superficial dorsal horn, strong in lamina I and weaker in lamina II. B, higher magnification view of the boxed region in A. Somatic staining is strong in lamina I. Numerous large puncta (arrows) and some processes (arrowheads) stain densely in lamina I. In lamina II, weakly sGC-positive somata lie among a dense plexus of stained processes. C, parasagittal sections; stained neurons in lamina I were elongated along the rostro-caudal axis. Immunopositive processes also run rostro-caudally in laminae I and IIo, but more randomly in deeper laminae. D, a positive soma in lamina I (arrowheads pointing to DAB deposits scattered in cytoplasm). A few positive dendrites are also visible (arrows). E, transverse section of a positive dendrite located in lamina I. F, a stained axonal terminal makes synaptic contact onto a dendrite in lamina IIi. Scale bars = 100 μm (A); 20 μm (B, C); 2 μm (D); 0.5 μm (E, F).
Cellular expression of sGCβ1
The superficial dorsal horn comprises both projection neurons and local interneurons. To determine whether projection neurons express sGC, we performed double labeling with NK1 receptor, a marker for the large majority of ascending projection neurons in this region (Todd et al., 2000). NK1 receptor staining was predominantly in lamina I. Immunostaining outlined somata and dendrites, in a pattern suggesting selective staining of the plasma membrane. Nearly all NK1 receptor-positive cells in lamina I was strongly positive for sGCβ1, as were many NK1 receptor-stained processes (Fig. 3).
Fig. 3.

Double labeling of sGCβ1 with NK1 receptor. Boxed area in A is enlarged in B-D. NK1 receptor-positive somata and dendrites are mainly in lamina I. All NK1 receptor-positive cell bodies contain sGCβ1 staining (arrows). Many of the NK1 receptor-stained processes also stain for sGC (arrowheads). Scale bars = 50 μm (A); 10 μm (B-D).
To determine whether inhibitory interneurons express sGCβ1, we also performed double labeling with GABA (Fig. 4). GABA-positive soma were scattered throughout superficial dorsal horn; most of them also stained for sGC (asterisks, Fig. 4B). Since virtually all glycinergic interneurons in this region also contain GABA (Todd and Sullivan, 1990), this result suggests that sGC is expressed in most inhibitory interneurons. However, some sGC-positive neurons in lamina II were negative for GABA (arrows, Fig. 4C). Considering their laminar localization, it seems likely that these sGC-positive/GABA-negative neurons are excitatory interneurons. Notwithstanding previous studies suggesting that sGC may be present in astrocytes in brain (Nakane et al., 1983; Bellamy et al., 2000), we were unable to confirm this in spinal cord, using double-labeling with GFAP (data not shown).
Fig. 4.
Double labeling of sGCβ1 (green) with GABA (red) in transverse section. A, GABA-stained cell bodies scattered in superficial dorsal horn; the large majority are also stained for sGC. High magnification view of boxed areas in A are shown in B and C. B, three GABA-positive neurons are also immunopositive for sGC (asterisks). C, two sGC-positive cells in lamina II do not express GABA (arrows). Scale bars = 50 μm (A); 10 μm (B and C).
Lack of sGCβ1 in C fibers
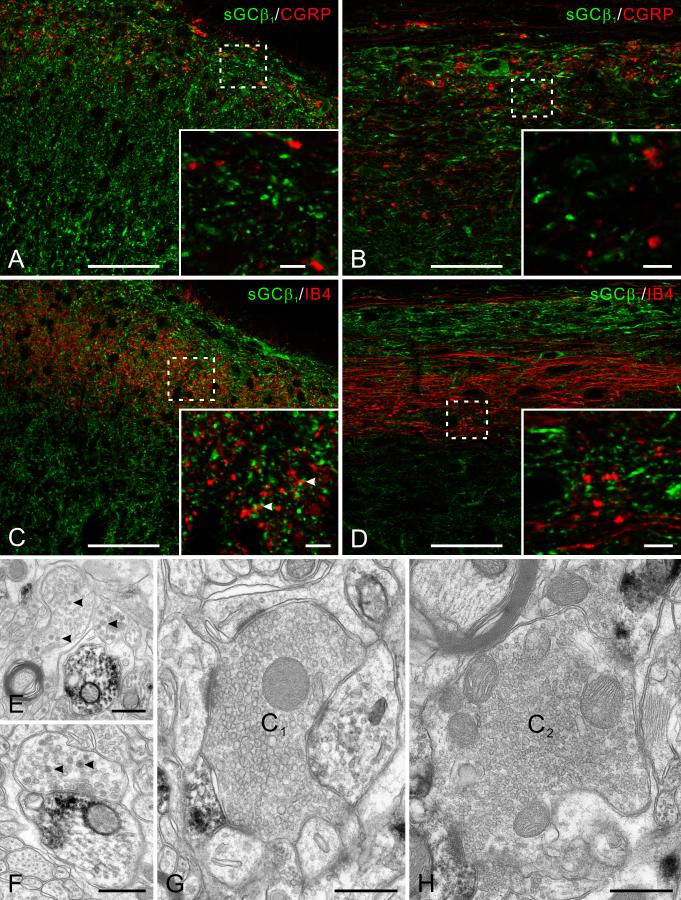
NO may modulate pain processing by acting on nociceptive primary afferent fibers to modify transmitter release (Garry et al., 1994; Aimar et al., 1998). We therefore examined whether sGCβ1 is present in these fibers, by performing double labeling immunofluorescence with CGRP or IB4, two markers widely used to label peptidergic and non-peptidergic unmyelinated primary afferents, respectively. CGRP staining was mainly in laminae I and IIo, whereas IB4 staining was mainly in lamina IIi, as previously reported (Belyantseva and Lewin, 1999). sGC only rarely colocalized with either CGRP (Fig. 5A, B) or IB4 (Fig. 5C, D), leading us to conclude that unmyelinated primary afferent fibers contain little or no sGC.
Fig. 5.
The relationship of sGC with primary afferent fibers. A-D: Double labeling of sGCβ1 (green) with two markers for unmyelinated primary afferent fibers, CGRP and IB4 (red) on transverse (A and C) and parasagittal (B and D) sections. Insets are high magnification view of the boxed areas. A and B, CGRP-stained primary afferents terminate mainly in lamina I and IIo. No colocalization of sGC with CGRP was found in transverse or parasagittal sections. C and D, IB4-stained (non-peptidergic) primary afferents terminate mainly in lamina IIi. sGC and IB4 staining are in general unrelated both in transverse and parasagittal section. Occasional contacts are visible (arrowheads), but no clear colocalization. E-H: sGCβ1 immunoperoxidase staining at EM level showing lack of sGC in primary afferent terminals. E, two immunonegative non-glomerular terminals in lamina I that contain dense-core vesicles (arrowheads) close to an immunopositive dendrite. F, an immunonegative terminal (likely to be peptidergic for its content of dense-core vesicles, arrowheads) makes synaptic contact onto an immunopositive dendrite. G, an immunonegative central terminal of type I (C1) synaptic glomerulus in lamina II. H, a negative central terminal of type II (C2) synaptic glomerulus in lamina II. Scale bars = 50 μm (A-D); 5 μm (insets of A-D); 0.5 μm (E-H).
We further examined this issue at the EM level. A small fraction of axon terminals were immunopositive for sGCβ1 (Fig. 2F), but the morphology of these terminals was not typical of primary afferents terminating in this region (Bernardi et al., 1995). Morphologically-identifiable primary afferent terminals were always immunonegative for sGCβ1. These include non-glomerular terminals that contain numerous dense core vesicles (Fig. 5E, F) and the central terminals of both type I and type II glomeruli (Fig. 5G, H). Thus, the EM data are consistent with our conclusion from immunofluorescence that primary afferent fibers lack sGC.
The relationship of sGC and nNOS
To investigate the spatial relationship between sources and targets of NO in superficial dorsal horn, we performed immunofluorescent double-labeling for sGCβ1 and nNOS (Fig. 6). The overall pattern of nNOS staining was consistent with previous reports (Valtschanoff et al., 1992; Saito et al., 1994). NOS-positive neurons were scattered in superficial dorsal horn, concentrating in deep lamina IIi (Fig. 6A). NOS-stained processes and puncta formed a dense plexus in lamina IIi, and a moderately dense plexus in lamina I, whereas staining in lamina IIo was sparse.
Fig. 6.
The spatial relationship of sGCβ1 and nNOS. nNOS-positive cell bodies are scattered in superficial dorsal horn, most numerous at the ventral border of lamina II. One nNOS-positive cell body is also stained for sGCβ1 (arrow in A). nNOS-positive processes form a dense plexus in lamina IIi, lamina I has moderate nNOS immunoreactivity, and lamina IIo has little. B-D are enlargements of boxed areas in A showing the relationship between the two antigens in different laminae. nNOS-positive puncta are commonly closely apposed to sGC-positive puncta; some of these are circled. Occasional apparent colocalization is also visible (arrowhead). E: Triple labeling with synaptophysin. A few nNOS/sGC contacts (circles) are associated with synaptophysin (arrowheads), while most of those contacts are not. Scale bars = 50 μm (A); 10 μm (B-E).
nNOS and sGC were generally expressed in different cells, though occasional double-labeled cells were seen (arrow, Fig. 6A). At high magnification, nNOS-positive processes and puncta throughout the superficial dorsal horn often lay very close to sGC-positive structures, or made contact with them (Fig. 6B-D). Colocalization of the two antigens in the same subcellular structures was occasionally detected (arrowhead in C), but apposition was much commoner. Analysis of 178 nNOS-positive puncta showed that 114 (64%) contacted sGC-positive puncta, whereas 26 (15%) overlapped or completely colocalized with sGC-positive puncta; only 38 (21%) failed to exhibit a clear relationship with sGC-positive profiles. To determine whether contacts between NOS-positive and sGC-positive puncta are synaptic, we performed triple labeling with synaptophysin, finding that synaptophysin colocalized with either nNOS or sGC puncta in ∼1/3 of cases (94 of 294 NOS/sGC contacts). Of these synaptic contacts, ∼70% of the synaptophysin-positive puncta contained nNOS (66 of 94), and the remainder contained sGC. Thus, much of the influence of NO on sGC in this region may involve nonsynaptic dendrodendritic interactions (Fig. 6E).
DISCUSSION
sGCβ1 in the spinal cord
Message for sGC subunits has been reported in the spinal cord: α1 and β1 mRNAs were detected in human spinal cord (Budworth et al., 1999); both α1 and α2 mRNAs were detected in mouse spinal cord (Liang and Clark, 2004); and α1, α2 and β1 (but not β2) mRNAs were detected in rat spinal cord (Okamoto, 2004). sGC enzymatic activity has also been demonstrated in superficial dorsal horn, using cGMP immunostaining after incubation of the sections with NO donors (Morris et al., 1994; Vles et al., 2000). However, a previous study found α1 protein in rat spinal cord, but not β1 (Tao and Johns, 2002), contrary to the present result. The discrepancy may reflect differences in antibodies used in the two studies. The previous study performed Western blot analysis using a polyclonal antibody thought to recognize both α1 and β1 subunits. However, this antibody apparently detects the α1 subunit preferentially, since in lung, which contains similar levels of the two subunits (Kamisaki et al., 1986), the band corresponding to β1 subunit was much weaker than the α1 band (see Fig. 1A in Tao and Johns, 2002). In contrast, the antibody used in the present study is β1-specific, detecting a single band corresponding to the β1 subunit throughout the rat brain (whole brain, Ding et al., 2004; cortex, hippocampus, cerebellum, unpublished data). It seems unlikely that β2 is the main β subunit in superficial dorsal horn, since β2 mRNA was not detected in spinal cord (Okamoto, 2004); moreover, we are not aware of published evidence for β2 protein expression in mammalian tissue (Koesling et al., 2004). Our data show that β1 is expressed at substantial levels in spinal cord. Enzymatically-active sGC is a heterodimer (Zabel et al., 1999); since we previously demonstrated massive colocalization of α and β subunits (Ding et al., 2004), the present immunohistochemical data are likely to detect the sites of functional enzyme.
sGC and spinal neurons
Combining our data with previous information, we summarize the NO-cGMP pathway in the superficial dorsal horn in Fig. 7. sGC was expressed at high levels in nearly all NK1 receptor-expressing cells in lamina I, representing the large majority of projection neurons in this lamina (Todd et al., 2000). nNOS staining forms a dense plexus in lamina I; thus, NO is well-positioned to influence those projection neurons directly via cGMP. These NK1 receptor-positive projection neurons, which may be the most functionally-significant target of NO, receive massive synaptic input from peptidergic C fibers that release substance P (SP) to activate NK1 receptor (Todd, 2002). That these neurons play a pivotal role in pain processing is supported by experiments showing that their selective ablation markedly attenuates response to noxious stimuli (Mantyh et al., 1997). We also found weakly sGC-positive local circuit neurons, mostly in lamina II. Since both excitatory and inhibitory interneurons are potential targets of NO, its net effect may be complex, perhaps explaining the variable effects of NO found in different experimental pain models (see review by Luo and Cizkova, 2000). Colocalization of sGC and nNOS was infrequent, suggesting that NO acts in a paracrine manner, though it is not clear from our data whether these interactions are predominantly at synapses.
Fig. 7.
Schematic diagram of the NO-cGMP pathway in superficial dorsal horn. nNOS-positive cells, lying mainly in deep lamina II, produce NO when activated, which diffuses to act on sGC-expressing structures. Concentration of sGC is indicated by darkness of shading. NK1 receptor-expressing projection neurons (black) contain high levels of sGC, and thus may be the primary target of NO. Excitatory and inhibitory interneurons are also potential targets of NO. Unmyelinated primary afferents, which lack sGC, are not affected by NO via the cGMP pathway.
sGC and nociceptive primary afferent fibers
We did not detect sGC in unmyelinated primary afferent terminals, at variance with the conclusions of a previous study (Maihofner et al., 2000). That study provided indirect evidence that the sGC-positive fibers were of primary afferent origin, whereas we provide direct evidence, using both double-labeling immunofluorescence and electron microscopy, that primary afferents lack sGC. However, we cannot exclude that the discrepancy reflects a species difference between rats and mice, especially since the expression patterns of sGC may differ between rat and mouse hippocampus (van Staveren et al., 2004). It also remains possible that C fibers contain sGC at extremely low levels, or in a form our antibody does not detect.
It has been suggested that NO acts retrogradely on primary afferent terminals to increase release of neuropeptides, thus explaining its role in chronic pain (Aimar et al., 1998). However, a study showing that neither NO nor cGMP altered neuropeptide release from cultured sensory neurons (Dymshitz and Vasko, 1994) calls this hypothesis into question. The NO donor sodium nitroprusside can evoke the release of SP and CGRP from spinal cord slices (Garry et al., 1994), but it was not shown that primary afferents are direct targets of NO. Our data suggest instead that this effect arises indirectly, via actions on local circuit neurons (though we cannot exclude an effect of NO on primary afferent fibers via a pathway independent of cGMP).
Besides C fibers, nociceptive stimuli are also conducted by some thinly myelinated Aδ fibers, which mediate the rapid first phase of pain (Kajander and Bennett, 1992; Millan, 1999). The lack of sGC in type II glomerular terminals, thought to arise from Aδ fibers (Coimbra et al., 1984; Bernardi et al., 1995), suggests that also Aδ fibers are not direct retrograde targets of NO. However, a previous study suggests that Aδ fibers provide direct synaptic input to nNOS-expressing neurons in superficial dorsal horn (Bernardi et al., 1995). In combination with previous data (Kajander and Bennett, 1992), our results lead to the hypothesis that NO produced in response to abnormal activity in Aδ fibers at the onset of pain may sensitize the NK1 receptor-expressing projection neurons, leading to pathological pain.
ACKNOWLEDGMENTS
We thank Susan Grand for Western blotting and Kristen Phend for EM support, and Alain Burette, Juli Valtschanoff, Aldo Rustioni, Chun-Rong Lu and Helen Willcockson for advice. JDD performed the experiments and prepared the plates; RJW guided the work and provided support.
Grant sponsor: National Institutes of Health; Grant number: NS35527 (to R.J.W.)
LITERATURES CITED
- Aimar P, Pasti L, Carmignoto G, Merighi A. Nitric oxide-producing islet cells modulate the release of sensory neuropeptides in the rat substantia gelatinosa. J Neurosci. 1998;18:10375–10388. doi: 10.1523/JNEUROSCI.18-24-10375.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy TC, Garthwaite J. The receptor-like properties of nitric oxide-activated soluble guanylyl cyclase in intact cells. Mol Cell Biochem. 2002;230:165–176. [PubMed] [Google Scholar]
- Bellamy TC, Wood J, Goodwin DA, Garthwaite J. Rapid desensitization of the nitric oxide receptor, soluble guanylyl cyclase, underlies diversity of cellular cGMP responses. Proc Natl Acad Sci U S A. 2000;97:2928–2933. doi: 10.1073/pnas.97.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–468. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bernardi PS, Valtschanoff JG, Weinberg RJ, Schmidt HHHW, Rustioni A. Synaptic interactions between primary afferent terminals and GABA and nitric oxide-synthesizing neurons in superficial laminae of the rat spinal cord. J Neurosci. 1995;15:1363–1371. doi: 10.1523/JNEUROSCI.15-02-01363.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budworth J, Meillerais S, Charles I, Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun. 1999;263:696–701. doi: 10.1006/bbrc.1999.1444. [DOI] [PubMed] [Google Scholar]
- Burette A, Zabel U, Weinberg RJ, Schmidt HHHW, Valtschanoff JG. Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J Neurosci. 2002;22:8961–8970. doi: 10.1523/JNEUROSCI.22-20-08961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Lee WT, Carlton SM. The effects of dorsal rhizotomy and spinal cord isolation on calcitonin gene-related peptide-labeled terminals in the rat lumbar dorsal horn. Neurosci Lett. 1988;90:27–32. doi: 10.1016/0304-3940(88)90781-1. [DOI] [PubMed] [Google Scholar]
- Coimbra A, Ribeiro-da-Silva A, Pignatelli D. Effects of dorsal rhizotomy on the several types of primary afferent terminals in laminae I-III of the rat spinal cord. An electron microscope study. Anat Embryol (Berl) 1984;170:279–287. doi: 10.1007/BF00318731. [DOI] [PubMed] [Google Scholar]
- Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Devoto SH, Barnstable CJ. SVP38: A synaptic vesicle protein whose appearance correlates closely with synaptogenesis in the rat nervous system. Annals of the N Y Acad of Sci. 1987;493:493–496. [Google Scholar]
- Ding JD, Burette A, Nedvetsky PI, Schmidt HHHW, Weinberg RJ. Distribution of soluble guanylyl cyclase in the rat brain. J Comp Neurol. 2004;472:437–448. doi: 10.1002/cne.20054. [DOI] [PubMed] [Google Scholar]
- Ding JD, Burette A, Weinberg RJ. Expression of soluble guanylyl cyclase in rat cerebral cortex during postnatal development. J Comp Neurol. 2005;485:255–265. doi: 10.1002/cne.20494. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Forstermann U, Tseng LF. Nitric oxide synthase immunoreactivity in rat spinal cord. Neurosci Lett. 1992;147:217–220. doi: 10.1016/0304-3940(92)90599-3. [DOI] [PubMed] [Google Scholar]
- Dymshitz J, Vasko MR. Nitric oxide and cyclic guanosine 3',5'-monophosphate do not alter neuropeptide release from rat sensory neurons grown in culture. Neuroscience. 1994;62:1279–1286. doi: 10.1016/0306-4522(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Eshkind LG, Leube RE. Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell Tissue Res. 1995;282:423–433. doi: 10.1007/BF00318874. [DOI] [PubMed] [Google Scholar]
- Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14:4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gibb BJ, Wykes V, Garthwaite J. Properties of NO-activated guanylyl cyclases expressed in cells. Br J Pharmacol. 2003;139:1032–1040. doi: 10.1038/sj.bjp.0705318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Son H, Arancio O. Nitric oxide as a retrograde messenger during long-term potentiation in hippocampus. Prog Brain Res. 1998;118:155–172. doi: 10.1016/s0079-6123(08)63206-9. [DOI] [PubMed] [Google Scholar]
- Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- Inoue T, Mashimo T, Shibuta S, Yoshiya I. Intrathecal administration of a new nitric oxide donor, NOC-18, produces acute thermal hyperalgesia in the rat. J Neurol Sci. 1997;153:1–7. doi: 10.1016/s0022-510x(97)00188-3. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in Aβ and Aδ primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Saheki S, Nakane M, Palmieri JA, Kuno T, Chang BY, Waldman SA, Murad F. Soluble guanylate cyclase from rat lung exists as a heterodimer. J Biol Chem. 1986;261:7236–7241. [PubMed] [Google Scholar]
- Kawamata T, Omote K. Activation of spinal N-methyl-D-aspartate receptors stimulates a nitric oxide/cyclic guanosine 3,5-monophosphate/glutamate release cascade in nociceptive signaling. Anesthesiology. 1999;91:1415–1424. doi: 10.1097/00000542-199911000-00035. [DOI] [PubMed] [Google Scholar]
- Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: structure and regulation. Neurochem Int. 2004;45:813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Eisenach JC, Figueroa JP. NMDA causes release of nitric oxide from rat spinal cord in vitro. Brain Res. 1994;637:287–291. doi: 10.1016/0006-8993(94)91246-7. [DOI] [PubMed] [Google Scholar]
- Liang DY, Clark JD. Modulation of the NO/CO-cGMP signaling cascade during chronic morphine exposure in mice. Neurosci Lett. 2004;365:73–77. doi: 10.1016/j.neulet.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Lin Q, Peng YB, Wu J, Willis WD. Involvement of cGMP in nociceptive processing by and sensitization of spinothalamic neurons in primates. J Neurosci. 1997;17:3293–3302. doi: 10.1523/JNEUROSCI.17-09-03293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Wu J, Peng YB, Cui M, Willis WD. Nitric oxide-mediated spinal disinhibition contributes to the sensitization of primate spinothalamic tract neurons. J Neurophysiol. 1999;81:1086–1094. doi: 10.1152/jn.1999.81.3.1086. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Cizkova D. The role of nitric oxide in nociception. Curr Rev Pain. 2000;4:459–466. doi: 10.1007/s11916-000-0070-y. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Euchenhofer C, Tegeder I, Beck KF, Pfeilschifter J, Geisslinger G. Regulation and immunhistochemical localization of nitric oxide synthases and soluble guanylyl cyclase in mouse spinal cord following nociceptive stimulation. Neurosci Lett. 2000;290:71–75. doi: 10.1016/s0304-3940(00)01302-1. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993;54:291–300. doi: 10.1016/0304-3959(93)90028-N. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Morris R, Southam E, Gittins SR, de Vente J, Garthwaite J. The NO-cGMP pathway in neonatal rat dorsal horn. Eur J Neurosci. 1994;6:876–879. doi: 10.1111/j.1460-9568.1994.tb00998.x. [DOI] [PubMed] [Google Scholar]
- Nakane M, Ichikawa M, Deguchi T. Light and electron microscopic demonstration of guanylate cyclase in rat brain. Brain Res. 1983;273:9–15. doi: 10.1016/0006-8993(83)91088-0. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Molecular cloning of a novel variant of the rat soluble guanylate cyclase β2 subunit. Int J Biochem Cell Biol. 2004;36:472–480. doi: 10.1016/j.biocel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Osborne MG, Coderre TJ. Effects of intrathecal administration of nitric oxide synthase inhibitors on carrageenan-induced thermal hyperalgesia. Br J Pharmacol. 1999;126:1840–1846. doi: 10.1038/sj.bjp.0702508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Eliasson C, Chien CL, Kindblom LG, Liem R, Hamberger A, Betsholtz C. GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp Cell Res. 1998;239:332–343. doi: 10.1006/excr.1997.3922. [DOI] [PubMed] [Google Scholar]
- Saito S, Kidd GJ, Trapp BD, Dawson TM, Bredt DS, Wilson DA, Traystman RJ, Snyder SH, Hanley DF. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994;59:447–456. doi: 10.1016/0306-4522(94)90608-4. [DOI] [PubMed] [Google Scholar]
- Schmidt HHHW, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Roth KA. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem. 1996;44:1331–1335. doi: 10.1177/44.11.8918908. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johns RA. Activation and up-regulation of spinal cord nitric oxide receptor, soluble guanylate cyclase, after formalin injection into the rat hind paw. Neuroscience. 2002;112:439–446. doi: 10.1016/s0306-4522(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Olesen J. Nitric oxide in primary headaches. Curr Opin Neurol. 2001;14:315–321. doi: 10.1097/00019052-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12:689–700. doi: 10.1046/j.1460-9568.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ, Rustioni A. NADPH diaphorase in the spinal cord of rats. J Comp Neurol. 1992;321:209–222. doi: 10.1002/cne.903210204. [DOI] [PubMed] [Google Scholar]
- van Staveren WC, Steinbusch HW, Markerink-van Ittersum M, Behrends S, de Vente J. Species differences in the localization of cGMP-producing and NO-responsive elements in the mouse and rat hippocampus using cGMP immunocytochemistry. Eur J Neurosci. 2004;19:2155–2168. doi: 10.1111/j.0953-816X.2004.03327.x. [DOI] [PubMed] [Google Scholar]
- Vigna SR, Bowden JJ, McDonald DM, Fisher J, Okamoto A, McVey DC, Payan DG, Bunnett NW. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994;14:834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vles JS, de Louw AJ, Steinbusch H, Markerink-van Ittersum M, Steinbusch HW, Blanco CE, Axer H, Troost J, de Vente J. Localization and age-related changes of nitric oxide- and ANP-mediated cyclic-GMP synthesis in rat cervical spinal cord: an immunocytochemical study. Brain Res. 2000;857:219–234. doi: 10.1016/s0006-8993(99)02434-8. [DOI] [PubMed] [Google Scholar]
- Willis WD. Long-term potentiation in spinothalamic neurons. Brain Res Brain Res Rev. 2002;40:202–214. doi: 10.1016/s0165-0173(02)00202-3. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following intradermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Zabel U, Hausler C, Weeger M, Schmidt HHHW. Homodimerization of soluble guanylyl cyclase subunits. Dimerization analysis using a glutathione s-transferase affinity tag. J Biol Chem. 1999;274:18149–18152. doi: 10.1074/jbc.274.26.18149. [DOI] [PubMed] [Google Scholar]