Abstract
Protein kinase C (PKC) is known to regulate phosphorylation of substrates such as MARCKS, GAP-43 and the NMDA receptor, all of which have been linked to synaptic plasticity underlying information storage processes. Here we report on 3 transgenic mice isoforms differentiated both by mutation of the PKC site on GAP-43 as well as by their performance in 3 learning situations: 1) a radial arm maze task, which evaluates spatial memory and its retention, 2) fear conditioning which assesses contextual memory and 3) the water maze which also evaluates spatial memory and its retention. The present results show, for the first time to our knowledge, that the phosphorylation state of a single site on an identified brain growth- and plasticity-associated protein differentially regulates performance of 3 different memory-associated tasks.
Post-translational modification of synaptic proteins already synthesized and located at pre- and post-synaptic sites has been proposed as a critical event leading to long-lasting information storage (Routtenberg, 1985; Routtenberg and Rekart, 2005; Routtenberg, 2008). The growth- and plasticity-associated protein, GAP-43, is developmentally regulated, concentrated in growth cones, excluded from dendrites and selectively transported to axons and their terminals. PKC regulates GAP-43 function by phosphorylation on its ser-41 in rat, mouse and man and ser-42 in chick (Tan and Parker, 2003). Phosphorylation of the PKC site is highly correlated with memory formation and synaptic plasticity of LTP (Benowitz and Routtenberg, 1997; Linden and Routtenberg, 1989; Perrone Bizzozero and Tanner, 2006; Skene et al., 1986; Sossin, 2007).
Here we report on 3 different transgenic mouse lines overexpressing GAP-43, two of which have mutations of the PKC phosphorylation site (Aigner et al., 1995). The G-Phos line overexpresses the phosphorylatable and dephosphorylatable form of chick GAP-43 (the native protein therefore, no mutation), the G-Perm line overexpresses chick GAP-43 that is permanently pseudophosphorylated as a consequence of the aspartate substitution for serine-42 and the G-NonP line overexpresses nonphosphorylatable GAP-43 in which alanine is substituted at the serine-42 site.
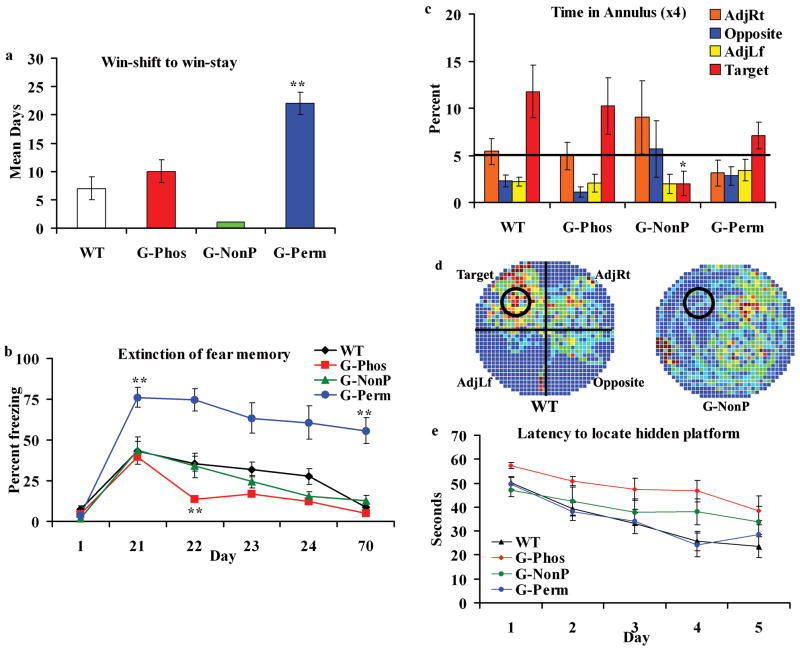
In the first task used, the radial arm maze, which is intended to evaluate spatial memory, it was reported that the 3 transgenic lines differed in their performance on the non-matching to sample (‘win-shift’) task with a 20 min delay between the first and second trials (see Routtenberg et al., 2000, for details). The G-Phos line showed significantly enhanced performance relative to the G-Perm and G-NonP lines. Here we report that the G-Perm group importantly differed from the other 2 when they were required to switch from a win-shift to a win-stay strategy unlearning the win-shift habit of entering arms that were previously unbaited and learning to return to the same spatial location where a single arm was baited. Acquisition of the win-stay habit was judged adequate when performance reached a criterion of 2 or fewer errors on 4 consecutive days (Routtenberg, et al., 2000). The measure of their ability to unlearn win-shift is shown in Figure 1A as the number of days to reach chance levels of performance on the win-stay task. The latter is a delayed matching to sample task while the former is a delayed nonmatching to sample task. The G-Perm group revealed a striking persistence in win-shift strategy 22 days after the change in task procedure to win-stay. This was significantly different from the mean of 7 days for the WT group and 10 days for the G-Phos group (one way ANOVA on days required to switch; F(3,16) = 55.93, p < 0.001; Scheffe post hoc comparisons, all groups vs G-Perm, p < 0.05).
Figure 1.
Protein kinase C site-specific differential performance on 3 behavioral tasks of 3 transgenic mouse lines overexpressing wild type, permanently phosphorylated or unphosphorylatable forms of the growth protein GAP-43. A. Radial arm maze win-stay task. Time in days required to unlearn win-shift and return to chance performance (see Text). The WTs and G-Phos performed at chance levels in 7–10 days whereas G-Perm animals required 22 days (**, p < 0.01). The chance performance in the G-NonP group 1 day after terminating the win-shift task indicates poor win-shift abilities. B. Contextual fear conditioning. Both the G-Phos and WT groups showed similar freezing levels during the first retention test (Day 21) but the G-Phos group showed less freezing on the second extinction test (Day 22) compared to WT (** p < 0.01). In contrast, the G-Perm group showed high levels of freezing 1) on the 21 day retention test (**, p < 0.01 vs. all groups) and 2) at 70 days after initial fear conditioning that was significantly greater than all groups (**, p < 0.01). C – E. Morris water maze. Spatial abilities tested in the 3 lines revealed a deficit in retention, but not performance, for the G-NonP group. During a probe test, G-NonP mice did not search selectively in the target area where the platform had been located during training. This is shown both in the percent time spent searching in each of the 4 annuli during the 60 s test (C) and the occupancy plot (D). E. The poor retention for the hidden platform was not due to poor initial acquisition measured as latency to locate the platform (in seconds).
In the second task employed, contextual fear conditioning (see Rekart et al., 2005, for methods), the G-Perm group (n = 7), in striking contrast to the other groups, showed the highest levels of freezing on Day 21 as well as the best retention scores indicated by the persistently high levels of freezing over the course of subsequent testing (Fig 1B). In contrast, the G-Phos group (n = 9), showed an accelerated rate of extinction differing from the other groups. Mice were given contextual fear conditioning (context-shock pairings, no tone) followed by a series of context alone extinction trials that began 3 weeks after training. G-Perm animals showed significantly higher levels of freezing during the first retention test than all other groups (one way ANOVA; F(3,30) = 5.97, p < 0.001; Scheffe post hoc comparisons, all groups vs G-Perm, p < 0.05) and a dramatic persistence of freezing during the subsequent 3 extinction trials even when tested at 70d after initial training (Fig 1B; one way ANOVA; F(3,30) = 16.24, p < 0.0001; Scheffe post hoc comparisons, all groups vs G-Perm, p < 0.05). As shown in Figure 1B, the G-Perm group continued to show elevated freezing (55%) compared to the Wt group (9%) 70 days after initial acquisition. This suggests that there is a highly persistent freezing response that lasts for more than 2 months in the G-Perm group. In contrast, we found that the G-Phos group showed a significantly enhanced extinction (Fig 1B; compare Day 21 and Day 22 for G-Phos group; t(8) = 5.01, p < 0.001; Day 22 one way ANOVA comparing all three lines; F(3,28) = 18.95, p < 0.001; Scheffe post hoc comparisons, all groups vs G-Phos, p < 0.05). G-NonP animals (n = 10) showed little difference from the wild type controls. In sum, inability to extinguish freezing behavior in the G-Perm animals and rapid extinction in the G-Phos mice points to the PKC site on GAP-43 as bidirectionally regulating performance in the contextual fear conditioning paradigm.
To determine whether the observed behavioral differences between the G-Perm and WT groups were based on differences in shock sensitivity, their responsiveness (flinch, jump, vocalization) to an incremental series of foot shocks was studied. There were no significant differences in the mean shock level to produce a flinch (WT: 0.075, G-Perm: 0.12; t-test, p = 0.27), a jump (WT: 0.4, G-Perm: 0.26; t-test, p = 0.22) or a vocalization (WT: 0.3, G-Perm: 0.36; t-test, p = 0.51). Thus, the shock sensitivity data provide little support for the hypothesis that the G-Perm group show persistent freezing due to greater sensitivity to shock.
The physiological mechanism for the unexpected 70d persistence of freezing in the G-Perm mice may be related to an LTP-like process. We have previously compared perforant path LTP in these 3 transgenic isoforms. In contrast to the G-Phos and G-NonP lines, which showed decrements to wild type levels within 2 hours after LTP induction, the G-Perm line showed a persistent, elevated potentiated response 4 hr after tetanus (see Fig 2C Routtenberg et al., 2000).
In the third task used, the Morris water maze (for methods, see Holahan et al., 2007), differential performance among the 3 transgenic lines was also revealed. Here the G-NonP mice (n = 6) distinguished themselves by their poor retention performance on the probe test relative to the other groups (Fig 1C and D). Mice were given 8 spaced trials to locate a fixed hidden platform over 5 days of training. It is critical to note that the G-NonP animals, like their transgenic cousins (G-Phos, n = 9; G-Perm, n = 8) demonstrated no significant differences in acquisition over the 5 days of training (Fig 1E; latencies: F(12,136)=1.35, p = 0.19; path length: F(12,136)=1.15, p = 0.33). Seven days after the last day of training, a 60 s probe test was administered (no platform present). As shown in Figure 1C, the wild type (n = 14), G-Phos and G-Perm groups showed a directed search of the area where the platform was located. In contrast, the G-NonP group showed little preference for the region where the platform was originally located (see occupancy plot in Fig 1D; one way ANOVA on percent time in target area; F(3,34) = 3.66, p < 0.05; Scheffe post hoc comparisons, all groups vs G-NonP, p < 0.05). Despite their ability to perform the task demands (as evidenced by their significant decrease in latencies from Day 1 to Day 5; p < 0.05), the G-NonP mice spent very little time searching in the platform location 1 week later.
While we can not exclude the possibility that the learning strategies employed by the G-NonP group were different than those of the WT group, there were no differences over days in path length swum to find the platform nor were there any differences in percent thigmotaxis between the two groups over the 5 acquisition days. Based on these metrics, it appears that the WT and G-NonP groups are carrying out the task demands using similar strategies.
The present findings are the first evidence to our knowledge that a single phosphorylation site on a particular protein can selectively yet pervasively regulate putative information storage processes. They demonstrate that the PKC site on GAP-43 differentially regulates the ability of transgenic mice to perform on 3 different memory-associated tasks. To provide a descriptive account of the ability of each strain one might characterize the a) G-Phos animals as possessing superior learning abilities evidenced in their enhanced behavioral flexibility, b) G-Perm mice as revealing a persistence of behavioral responding that leads to inflexibility and apparent difficulty in unlearning and c) G-NonP animals as having a selective retention deficit revealed in a task intended to test spatial memory ability.
The present results by themselves might warrant the concern that the difference among phenotypes is one of behavioral performance or motoric ability and does not reflect the role of GAP-43 in information storage processes. However, these findings should be considered in the context of work carried out over decades, which has indicated that this growth protein plays a critical role in the morphological and physiological remodeling of the nervous system under conditions when no behavior is assessed (for review see Benowitz and Routtenberg, 1997). Taken together with several biochemically-based reports demonstrating the bidirectional fact that 1) behavior regulates the phosphorylation state of this protein and 2) the phosphorylation state of the protein regulates behavior, such compelling converging lines of evidence leads us to conclude that the phosphorylation state of GAP-43 plays a central role in the regulation and control of memory formation processes.
In a study of the effects on behavior of a double mutation substituting alanine on two serine AMPA receptor phosphorylation sites, Lee, et al (2003) observed a mild acquisition impairment. This contrasts with the dramatic behavioral phenotypes seen in the present study when a single PKC site on GAP-43 is mutated so that serine substitution by either aspartate generates the ‘persistence’ of the G-Perm mice, or alanine, which gives rise to the G-NonP mnemonically-challenged strain. This leads us to suggest that in normal animals the regulation of the phosphorylation state of the PKC site on GAP-43 importantly regulates information storage in a potentially wide range of different learning experiences.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner H-R, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Honegger KS, Tabatadze N, Routtenberg A. GAP-43 gene expression regulates information storage. Learn Mem. 2007;14(6):407–15. doi: 10.1101/lm.581907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, et al. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112(5):631–43. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Routtenberg A. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Rev. 1989;14:279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Perrone Bizzozero N, Tanner DC. GAP-43 in Neural Development and Plasticity. In: Lim R, Lajtha A, editors. The Handbook of Neurochemistry and Molecular Neurobiology. 3. New York: Springer; 2006. [Google Scholar]
- Rekart JL, Meiri K, Routtenberg A. Hippocampal-dependent memory is impaired in heterozygous GAP-43 knockout mice. Hippocampus. 2005;15(1):1–7. doi: 10.1002/hipo.20045. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Phosphoprotein regulation of memory formation: enhancement and control of synaptic plasticity by protein kinase C and protein F1 (GAP-43) Ann N Y Acad Sci. 1985;444:203–11. doi: 10.1111/j.1749-6632.1985.tb37590.x. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. The substrate for long-lasting memory: if not protein synthesis, then what? Neurobiol Learn Mem. 2008;89(3):225–33. doi: 10.1016/j.nlm.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano PA, Namgung U. Enhanced learning after genetic overexpression of a brain growth factor. PNAS USA. 2000;97(13):7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28(1):12–9. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233(4765):783–6. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Sossin WS. Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem. 2007;14(4):236–46. doi: 10.1101/lm.469707. [DOI] [PubMed] [Google Scholar]
- Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376(Pt 3):545–52. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]