Abstract
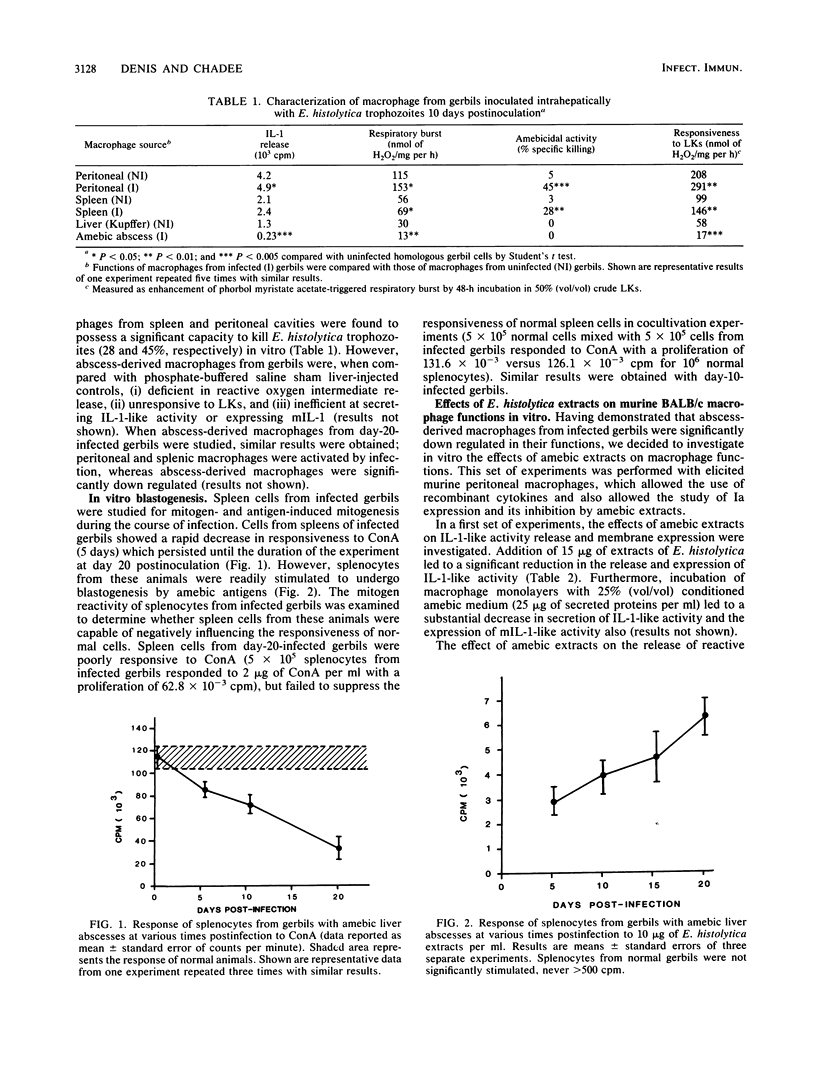
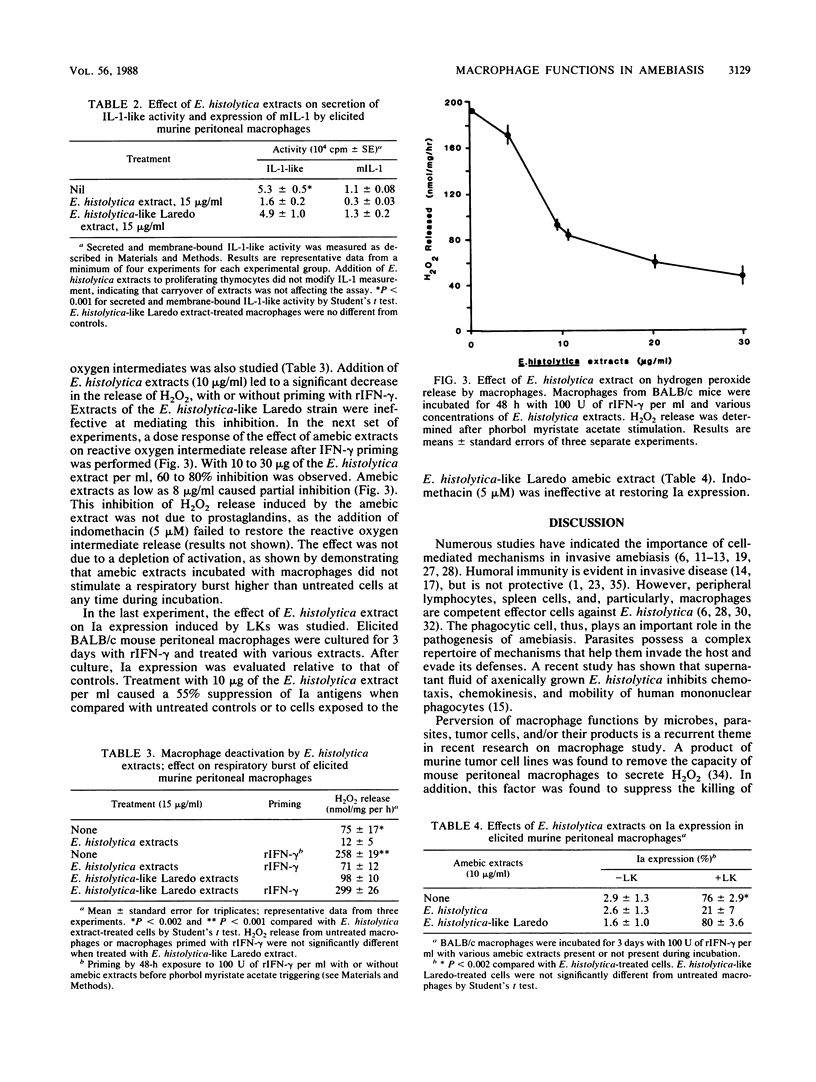
Experimental intrahepatic inoculation of the gerbil with Entamoeba histolytica trophozoites was used as a model of liver amebiasis to study the cellular immune response elicited by the parasite. It was shown that abscess-derived macrophages (5 to 20 days old) were deficient in their capacity to develop a respiratory burst, to secrete and express membrane-bound interleukin-1-like activity, and to kill E. histolytica trophozoites as well as to respond to lymphokines in vitro. However, macrophages isolated from the spleen and peritoneal cavities from the same infected animals were not significantly down regulated in these functions. Splenocytes from infected gerbils were shown to develop a strong responsiveness to amebic antigen, whereas their response to concanavalin A was suppressed. Crude E. histolytica extracts or conditioned medium down regulated murine BALB/c macrophage accessory and effector cell functions in vitro in a manner similar to abscess-derived macrophages, whereas crude extracts of the nonvirulent E. histolytica-like Laredo strain did not. Our results indicate that intrinsic or secreted products or both from E. histolytica are actively regulating macrophage functions at the abscess site and can possibly mediate other immunoregulatory mechanisms at distant targets.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust-Kettis A., Thorstensson R., Sundqvist K. G. Dynamics of the interaction between Entamoeba histolytica and components of the immune response. III. Fate of antibodies after binding to the cell surface. Scand J Immunol. 1981;13(5):473–481. doi: 10.1111/j.1365-3083.1981.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. Entamoeba histolytica: antibody responses and lymphoreticular changes in gerbils (Meriones unguiculatus) in response to experimental liver abscess and amebic extract infection. Z Parasitenkd. 1984;70(6):781–795. doi: 10.1007/BF00927131. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E., Moreau F. In vitro and in vivo interaction between trophozoites of Entamoeba histolytica and gerbil lymphoid cells. Infect Immun. 1985 Sep;49(3):828–832. doi: 10.1128/iai.49.3.828-832.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus). Am J Pathol. 1984 Oct;117(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Diamantstein T., Klos M., Gold D., Hahn H. Interaction between Entamoeba histolytica and the immune system. I. Mitogenicity of Entamoeba histolytica extracts for human peripheral T lymphocytes. J Immunol. 1981 Jun;126(6):2084–2086. [PubMed] [Google Scholar]
- Diamantstein T., Trissl D., Klos M., Gold D., Hahn H. Mitogenicity of Entamoeba histolytica extracts for murine lymphocytes. Immunology. 1980 Oct;41(2):347–352. [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E. In vitro amoebicidal activity of immune cells. Infect Immun. 1982 Apr;36(1):243–246. doi: 10.1128/iai.36.1.243-246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E., Kongshavn P. A. Role of macrophages in host defense against hepatic amoebiasis in hamsters. Infect Immun. 1983 Dec;42(3):1017–1019. doi: 10.1128/iai.42.3.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadirian E., Meerovitch E. Macrophage requirement for host defence against experimental hepatic amoebiasis in the hamster. Parasite Immunol. 1982 Jul;4(4):219–225. doi: 10.1111/j.1365-3024.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Huldt G., Davies P., Allison A. C., Schorlemmer H. U. Interactions between Entamoeba histolytica and complement. Nature. 1979 Jan 18;277(5693):214–216. doi: 10.1038/277214a0. [DOI] [PubMed] [Google Scholar]
- Kretschmer R. R. Immune phenomena in amebiasis. Surv Immunol Res. 1984;3(1):1–10. doi: 10.1007/BF02918589. [DOI] [PubMed] [Google Scholar]
- Kretschmer R., Collado M. L., Pacheco M. G., Salinas M. C., López-Osuna M., Lecuona M., Castro E. M., Arellano J. Inhibition of human monocyte locomotion by products of axenically grown E. histolytica. Parasite Immunol. 1985 Sep;7(5):527–543. doi: 10.1111/j.1365-3024.1985.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Krupp I. M., Powell S. J. Comparative study of the antibody response in amebiasis. Persistence after successful treatment. Am J Trop Med Hyg. 1971 May;20(3):421–424. doi: 10.4269/ajtmh.1971.20.421. [DOI] [PubMed] [Google Scholar]
- Nishihara T., Koga T., Hamada S. Suppression of murine macrophage interleukin-1 release by the polysaccharide portion of Haemophilus actinomycetemcomitans lipopolysaccharide. Infect Immun. 1988 Mar;56(3):619–625. doi: 10.1128/iai.56.3.619-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Zamacona G., Sepúlveda B., Capín N. R. Cell-mediated immunity in patients with amebic abscess of the liver. Clin Immunol Immunopathol. 1975 May;4(1):127–134. doi: 10.1016/0090-1229(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Pearson R. D., Symes P., Conboy M., Weiss A. A., Hewlett E. L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987 Oct 15;139(8):2749–2754. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Reed S. L., Sargeaunt P. G., Braude A. I. Resistance to lysis by human serum of pathogenic Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1983;77(2):248–253. doi: 10.1016/0035-9203(83)90083-4. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Ng W., McMaster W. R. Parasite-accessory cell interactions in murine leishmaniasis. II. Leishmania donovani suppresses macrophage expression of class I and class II major histocompatibility complex gene products. J Immunol. 1987 Mar 15;138(6):1926–1932. [PubMed] [Google Scholar]
- Reiner N. E. Parasite accessory cell interactions in murine leishmaniasis. I. Evasion and stimulus-dependent suppression of the macrophage interleukin 1 response by Leishmania donovani. J Immunol. 1987 Mar 15;138(6):1919–1925. [PubMed] [Google Scholar]
- Remaley A. T., Glew R. H., Kuhns D. B., Basford R. E., Waggoner A. S., Ernst L. A., Pope M. Leishmania donovani: surface membrane acid phosphatase blocks neutrophil oxidative metabolite production. Exp Parasitol. 1985 Dec;60(3):331–341. doi: 10.1016/0014-4894(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Martinez-Palomo A., Murray H. W., Conales L., Trevino N., Segovia E., Murphy C. F., Ravdin J. I. Patients treated for amebic liver abscess develop cell-mediated immune responses effective in vitro against Entamoeba histolytica. J Immunol. 1986 Apr 1;136(7):2633–2639. [PubMed] [Google Scholar]
- Salata R. A., Murray H. W., Rubin B. Y., Ravdin J. I. The role of gamma interferon in the generation of human macrophages cytotoxic for Entamoeba histolytica trophozoites. Am J Trop Med Hyg. 1987 Jul;37(1):72–78. doi: 10.4269/ajtmh.1987.37.72. [DOI] [PubMed] [Google Scholar]
- Salata R. A., Pearson R. D., Ravdin J. I. Interaction of human leukocytes and Entamoeba histolytica. Killing of virulent amebae by the activated macrophage. J Clin Invest. 1985 Aug;76(2):491–499. doi: 10.1172/JCI111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata R. A., Ravdin J. I. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. II. Mitogenic activity for human lymphocytes. J Infect Dis. 1985 May;151(5):816–822. doi: 10.1093/infdis/151.5.816. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Weinberg A., Borinsky R., Holt S. C., Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect Immun. 1988 Mar;56(3):589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern J. J., Graybill J. R., Drutz D. J. Murine amebiasis: the role of the macrophage in host defense. Am J Trop Med Hyg. 1984 May;33(3):372–380. doi: 10.4269/ajtmh.1984.33.372. [DOI] [PubMed] [Google Scholar]
- Szuro-Sudol A., Murray H. W., Nathan C. F. Suppression of macrophage antimicrobial activity by a tumor cell product. J Immunol. 1983 Jul;131(1):384–387. [PubMed] [Google Scholar]
- Szuro-Sudol A., Nathan C. F. Suppression of macrophage oxidative metabolism by products of malignant and nonmalignant cells. J Exp Med. 1982 Oct 1;156(4):945–961. doi: 10.1084/jem.156.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl D. Immunology of Entamoeba histolytica in human and animal hosts. Rev Infect Dis. 1982 Nov-Dec;4(6):1154–1184. doi: 10.1093/clinids/4.6.1154. [DOI] [PubMed] [Google Scholar]


