Abstract
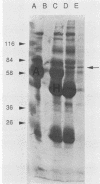
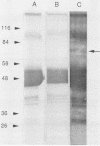
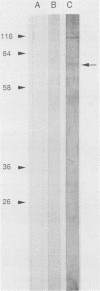
Circulating immune complexes are present in the sera of patients with visceral leishmaniasis caused by Leishmania donovani chagasi. In order to determine whether these complexes contain parasite antigens, sera were collected from Brazilian patients with visceral leishmaniasis and from hospitalized control subjects with other diagnoses. High-molecular-weight complexes were precipitated from pooled sera with 2.5% polyethylene glycol. Approximately 140-fold-more protein was precipitated from patient sera than from control sera; 12% of the total patient serum protein was precipitated. Patient serum precipitates contained immunoglobulins G (525 mg/dl), M (27 mg/dl), and A (8 mg/dl). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the patient serum precipitates revealed multiple bands, including a prominent band at 70 kilodaltons, that were not seen in precipitates of control sera. The 70-kilodalton band was recognized by human and hamster sera with antileishmanial antibodies, but not by control sera. Finally, immunization of BALB/c mice with the high-molecular-weight precipitates from patients elicited antileishmanial antibodies against L. donovani chagasi antigens as detected by enzyme-linked immunosorbent assay and Western blot (immunoblot) assay. In summary, sera of patients with American visceral leishmaniasis were found to have high-molecular-weight complexes that contained one or more parasite antigens. These complexes may play a role in the immunology of the disease, and detection of circulating parasite antigens has potential diagnostic importance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Badaró R., Jones T. C., Lorenço R., Cerf B. J., Sampaio D., Carvalho E. M., Rocha H., Teixeira R., Johnson W. D., Jr A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986 Oct;154(4):639–649. doi: 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Andrews B. S., Martinelli R., Dutra M., Rocha H. Circulating immune complexes and rheumatoid factor in schistosomiasis and visceral leishmaniasis. Am J Trop Med Hyg. 1983 Jan;32(1):61–68. doi: 10.4269/ajtmh.1983.32.61. [DOI] [PubMed] [Google Scholar]
- Casali P., Lambert P. H. Purification of soluble immune complexes from serum using polymethylmetacrylate beads coated with conglutinin or C1q. Application to the analysis of the components of in vitro formed immune complexes and of immune complexes occurring in vivo during leishmaniasis. Clin Exp Immunol. 1979 Aug;37(2):295–309. [PMC free article] [PubMed] [Google Scholar]
- DE Brito T., Hoshino-Shimizu S., Neto V. A., Duarte I. S., Penna D. O. Glomerular involvement in human kala-azar. A light, immunofluorescent, and electron microscopic study based on kidney biopsies. Am J Trop Med Hyg. 1975 Jan;24(1):9–18. [PubMed] [Google Scholar]
- Evans T., Reis M. de F., de Alencar J. E., Naidu T. G., de Jesus J. A., McAuliffe J. F., Pearson R. D. American visceral leishmaniasis (kala-azar). West J Med. 1985 Jun;142(6):777–781. [PMC free article] [PubMed] [Google Scholar]
- Flemmings B. J., Pappas M. G., Keenan C. M., Hockmeyer W. T. Immune complex decomplementation of canine sera for use in a complement-fixation test for diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 1984 Jul;33(4):553–559. doi: 10.4269/ajtmh.1984.33.553. [DOI] [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol Biochem Parasitol. 1988 Jan 1;27(1):53–62. doi: 10.1016/0166-6851(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Kager P. A., Hack C. E., Hannema A. J., Rees P. H., von dem Borne A. E. High C1q levels, low C1s/C1q ratios, and high levels of circulating immune complexes in kala-azar. Clin Immunol Immunopathol. 1982 Apr;23(1):86–93. doi: 10.1016/0090-1229(82)90073-3. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Rezai H. R., Fani M., Behforouz N. C. Evidence for the presence of circulating immune complexes in serum and C3b and C3d on red cells of kala-azar patients. Trans R Soc Trop Med Hyg. 1982;76(6):793–796. doi: 10.1016/0035-9203(82)90110-9. [DOI] [PubMed] [Google Scholar]
- Kohanteb J., Ardehali S. M., Rezai H. R. Detection of Leishmania donovani soluble antigen and antibody in the urine of visceral leishmaniasis patients. Trans R Soc Trop Med Hyg. 1987;81(4):578–580. doi: 10.1016/0035-9203(87)90414-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Kresina T. F. Network interactions in Schistosoma japonicum infection. Identification and characterization of a serologically distinct immunoregulatory auto-antiidiotypic antibody population. J Clin Invest. 1985 Dec;76(6):2338–2347. doi: 10.1172/JCI112245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Evans T., Wheeler D. A., Naidu T. G., De Alencar J. E., Davis J. S., 4th Humoral factors during South American visceral leishmaniasis. Ann Trop Med Parasitol. 1986 Aug;80(4):465–468. doi: 10.1080/00034983.1986.11812050. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Pearson R. D., de Alencar J. E., Romito R., Naidu T. G., Young A. C., Davis J. S., 4th Circulating immune complexes and rheumatoid factors in visceral leishmaniasis. J Infect Dis. 1983 Jun;147(6):1102–1102. doi: 10.1093/infdis/147.6.1102. [DOI] [PubMed] [Google Scholar]
- Pugin P., Miescher P. A. Le kala-azar. Etude clinique et physiopathologique à propos d'un nouveau cas observé en Suisse. Schweiz Med Wochenschr. 1979 Feb 24;109(8):265–269. [PubMed] [Google Scholar]
- Sartori A., De Oliveira A. V., Roque-Barreira M. C., Rossi M. A., Campos-Neto A. Immune complex glomerulonephritis in experimental kala-azar. Parasite Immunol. 1987 Jan;9(1):93–103. doi: 10.1111/j.1365-3024.1987.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Sehgal S., Aikat B. K., Pathania A. G. Immune complexes in Indian kala-azar. Bull World Health Organ. 1982;60(6):945–950. [PMC free article] [PubMed] [Google Scholar]
- Weisinger J. R., Pinto A., Velazquez G. A., Bronstein I., Dessene J. J., Duque J. F., Montenegro J., Tapanes F., de Rousse A. R. Clinical and histological kidney involvement in human kala-azar. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):357–359. doi: 10.4269/ajtmh.1978.27.357. [DOI] [PubMed] [Google Scholar]