Abstract
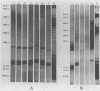
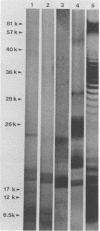
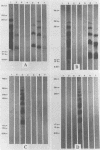
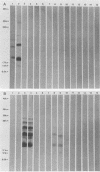
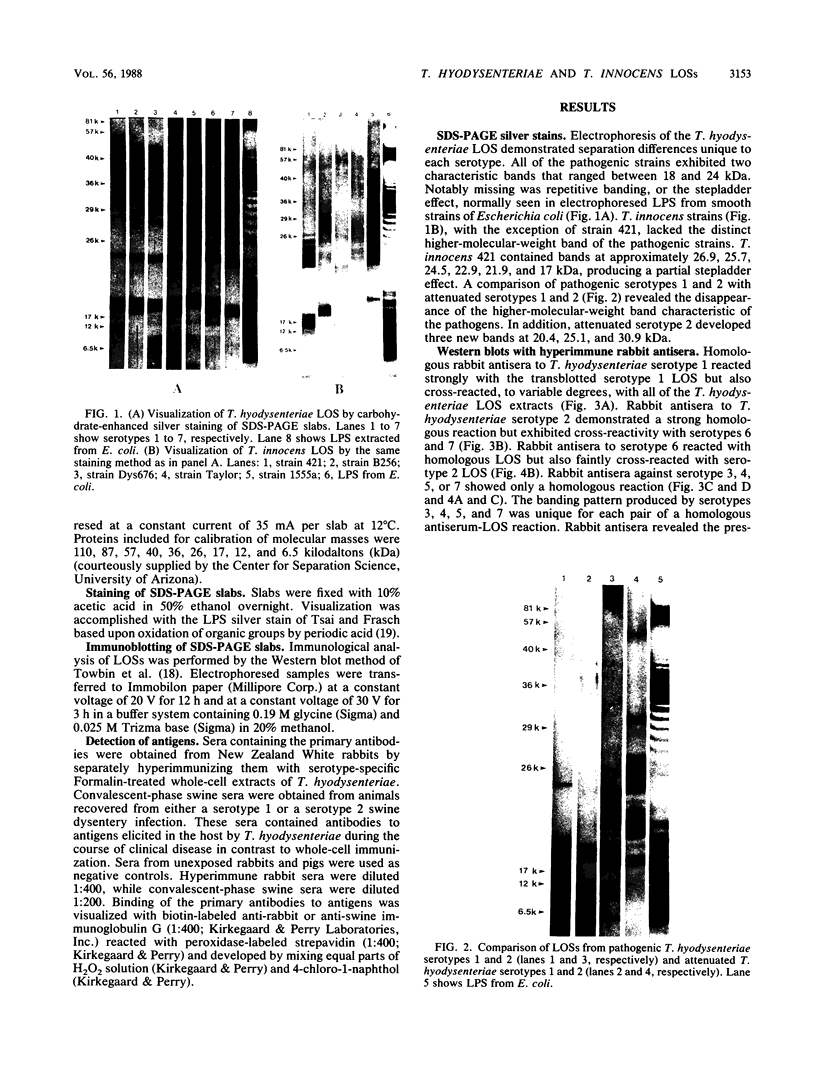
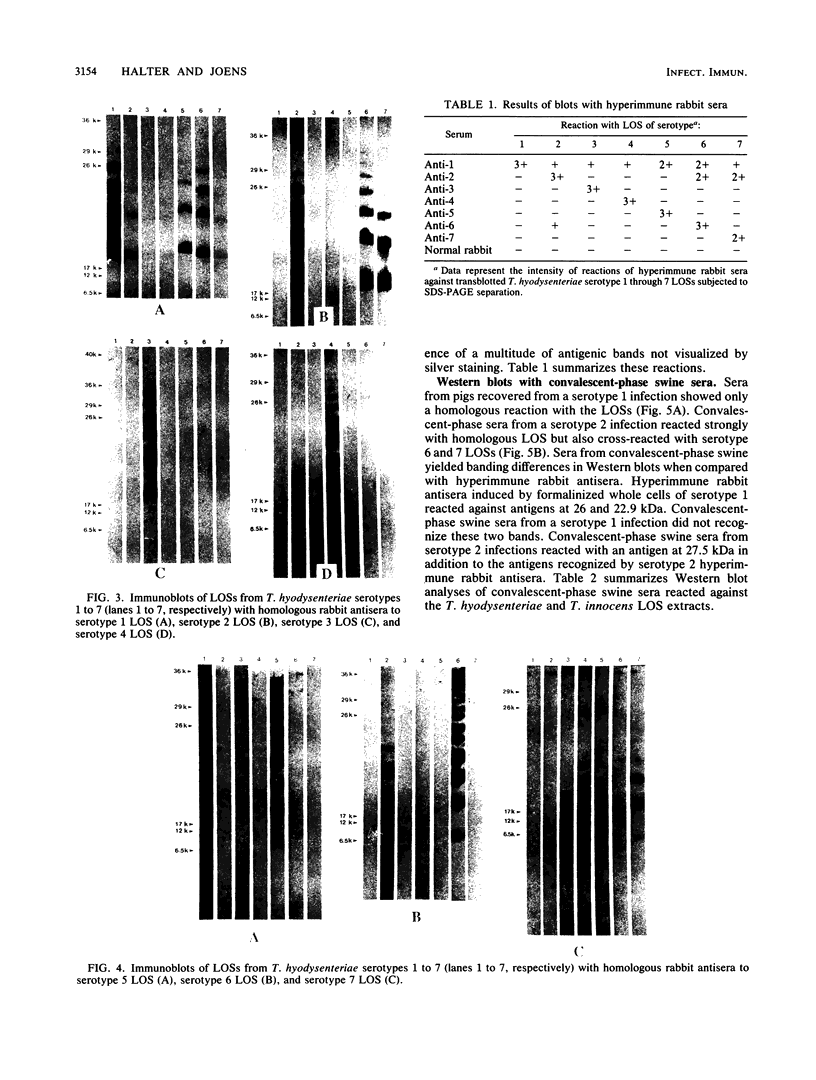
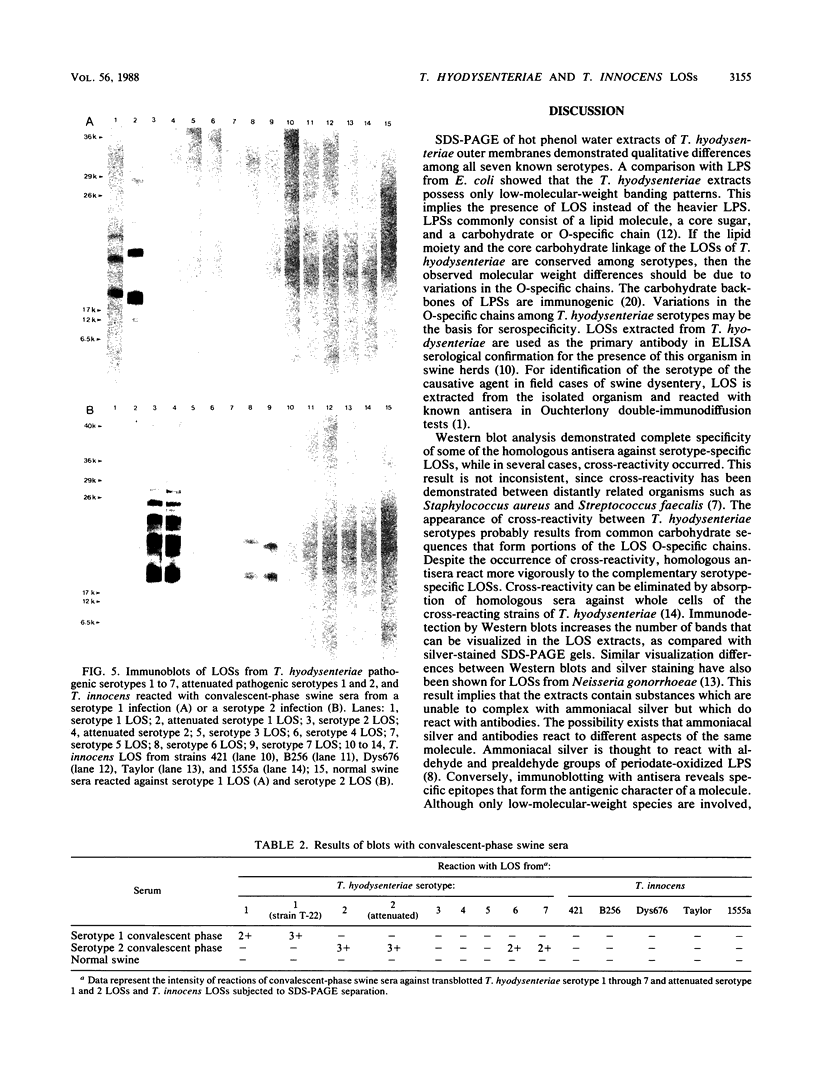
Lipooligosaccharides from Treponema hyodysenteriae serotypes 1 through 7, attenuated T. hyodysenteriae serotypes 1 and 2, and five strains of T. innocens were extracted with hot phenol water. The extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis separation and analyzed by lipopolysaccharide selective silver staining and Western blot (immunoblot) immunodetection. Silver staining revealed the presence of two bands that ranged between 18,000 and 24,000 daltons and that were serotype specific for T. hyodysenteriae. Attenuation of pathogenic strains resulted in the loss of the higher-molecular-weight band. Four of five T. innocens strains also lacked this particular band. T. innocens 421 had six bands between 17,000 and 26,900 daltons. Western blots with hyperimmune rabbit sera and convalescent-phase swine sera revealed antigenic variation among serotypes of T. hyodysenteriae and attenuated serotypes of T. hyodysenteriae. Convalescent-phase swine sera failed to recognize lipopolysaccharides from T. innocens. Differences in results obtained by lipopolysaccharide selective silver staining versus immunoblotting of the lipopolysaccharide preparations probably indicate that these two methods identify separate characteristics of the same molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Sandberg A. L., Rosentreich D. L. Comparison of the pyrogenicity, Limulus activity mitogenicity and complement reactivity of several bacterial endotoxins and related compounds. J Immunol. 1976 Oct;117(4):1238–1242. [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Heidelberger M. Cross-reactions of polysaccharides of staphylococci and streptococci in antipneumococcal and other antisera. Mol Immunol. 1984 Nov;21(11):1011–1013. doi: 10.1016/0161-5890(84)90109-3. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Glock R. D. Experimental infection in mice with Treponema hyodysenteriae. Infect Immun. 1979 Aug;25(2):757–760. doi: 10.1128/iai.25.2.757-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Nord N. A., Kinyon J. M., Egan I. T. Enzyme-linked immunosorbent assay for detection of antibody to Treponema hyodysenteriae antigens. J Clin Microbiol. 1982 Feb;15(2):249–252. doi: 10.1128/jcm.15.2.249-252.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A., Glock R. D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983 Aug;131(2):997–999. [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- Wilcock B. P., Olander H. J. Studies on the pathogenesis of swine dysentery. I. Characterization of the lesions in colons and colonic segments inoculated with pure cultures or colonic content containing Treponema hyodysenteriae. Vet Pathol. 1979 Jul;16(4):450–465. doi: 10.1177/030098587901600409. [DOI] [PubMed] [Google Scholar]
- Wilcock B. P., Olander H. J. Studies on the pathogenesis of swine dysentery. II. Search for a cytotoxin in spirochetal broth cultures and colon content. Vet Pathol. 1979 Sep;16(5):567–573. doi: 10.1177/030098587901600509. [DOI] [PubMed] [Google Scholar]