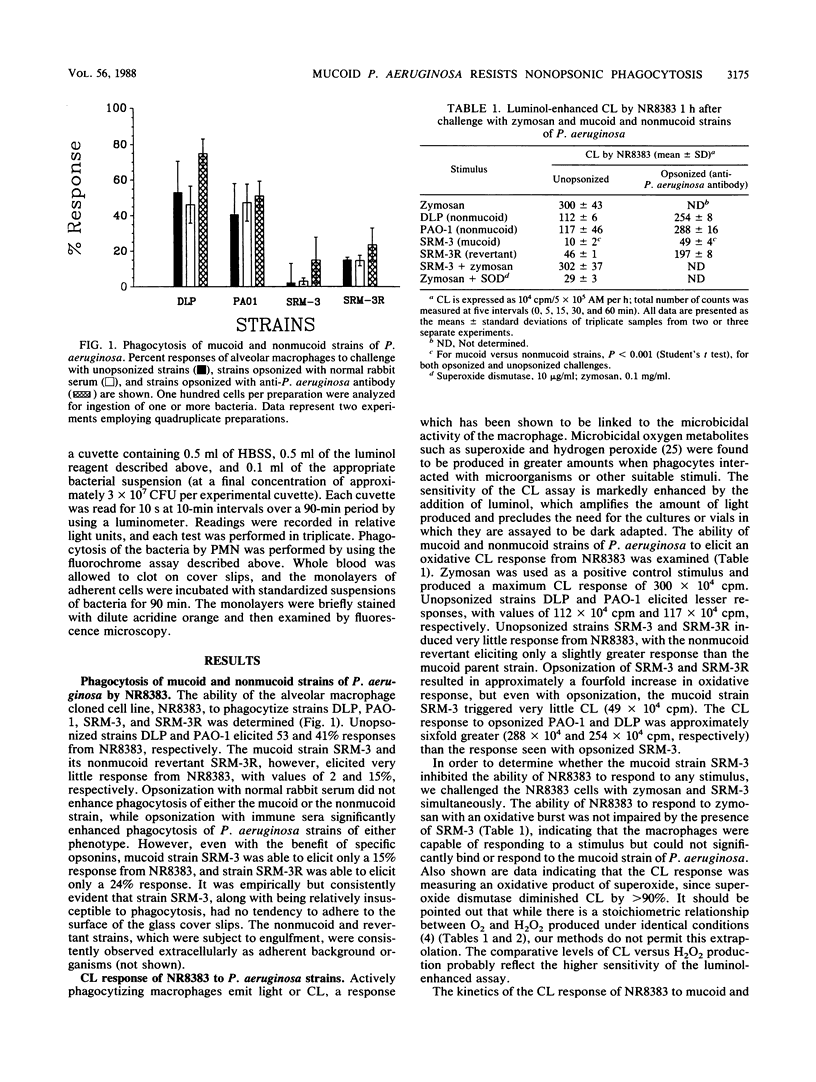
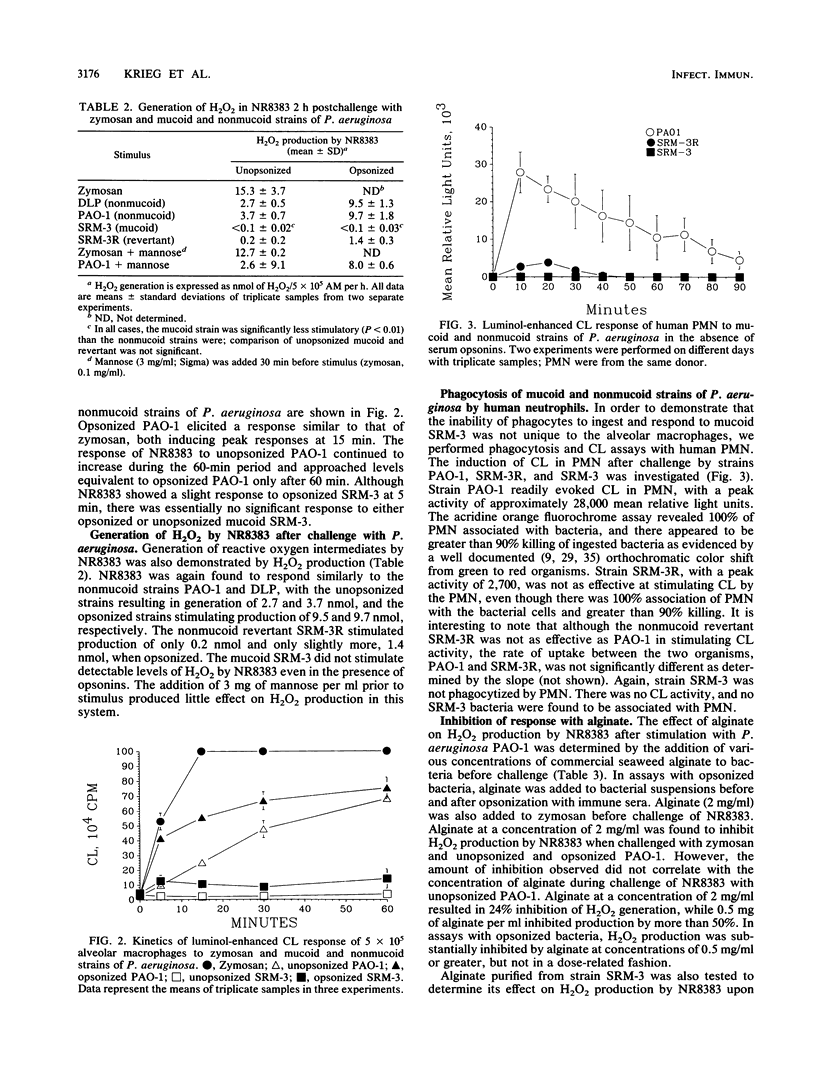
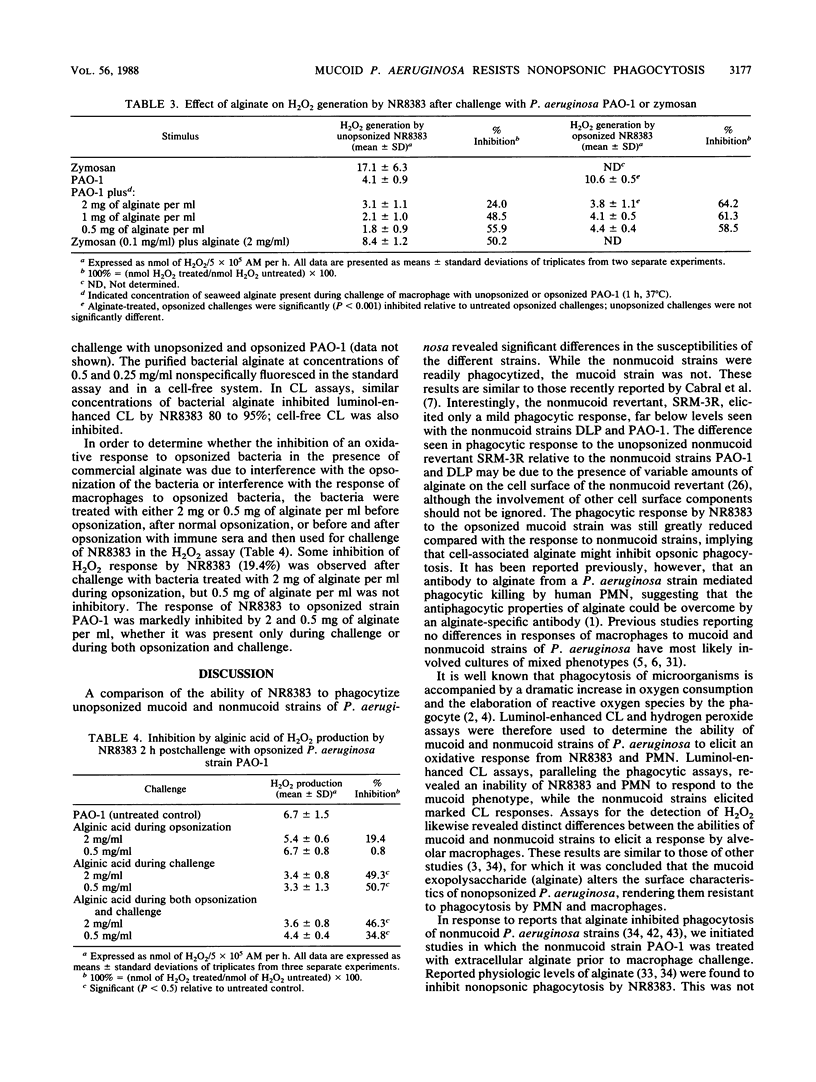
Abstract
A unique, recently described rat alveolar macrophage cell line (NR8383) was used to study the interaction of the pulmonary immune system with a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa (SRM-3), its nonmucoid revertant (SRM-3R), and a non-cystic fibrosis isolate (PAO-1). Strain SRM-3 was cultivated in a chemostat system to allow maintenance of an entirely mucoid population. The alveolar macrophage response to the mucoid and nonmucoid strains of P. aeruginosa was determined by visually quantitating phagocytosis in acridine orange-stained monolayers and measuring the induction of an oxidative burst as indicated by chemiluminescence and H2O2 production. In all experiments, fewer than 2% of the NR8383 cells engulfed the mucoid SRM-3 isolate, while SRM-3R and PAO-1 were phagocytized by 15 and 41%, respectively. Opsonization by normal serum (complement) provided minimal phagocytic enhancement of these strains, whereas specific anti-P. aeruginosa antibody slightly elevated phagocytic responses to strains with nonmucoid phenotypes while providing a sevenfold increase in uptake of SRM-3. Chemiluminescent and H2O2 responses were comparable with the levels of phagocytosis observed, with very little or no response to the mucoid strain SRM-3. The data indicate that the strains with mucoid phenotypes are refractile to ingestion and that studies which describe ingestion of mucoid strains were likely measuring ingestion of revertants. Alginic acid (2 mg/ml) was found to inhibit stimulation of macrophage response to the opsonized and unopsonized nonmucoid strain PAO-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., DesJardins D., Pier G. B. Opsonophagocytic killing activity of rabbit antibody to Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1985 Aug;49(2):281–285. doi: 10.1128/iai.49.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Bender J. G., Florman A. L., Van Epps D. E. Correlation of serum opsonic activity in cystic fibrosis with colonization and disease state: measurement of opsonins to Pseudomonas aeruginosa by neutrophil superoxide anion generation. Pediatr Res. 1987 Oct;22(4):383–388. doi: 10.1203/00006450-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Blackwood L. L., Pennington J. E. Influence of mucoid coating on clearance of Pseudomonas aeruginosa from lungs. Infect Immun. 1981 May;32(2):443–448. doi: 10.1128/iai.32.2.443-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral D. A., Loh B. A., Speert D. P. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987 Oct;22(4):429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Danley D. L., Hilger A. E. Stimulation of oxidative metabolism in murine polymorphonuclear leukocytes by unopsonized fungal cells: evidence for a mannose-specific mechanism. J Immunol. 1981 Aug;127(2):551–556. [PubMed] [Google Scholar]
- DeChatelet L. R., Mulikin D., McCall C. E. The generation of superoxide anion by various types of phagocyte. J Infect Dis. 1975 Apr;131(4):443–446. doi: 10.1093/infdis/131.4.443. [DOI] [PubMed] [Google Scholar]
- Di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (first of three parts). N Engl J Med. 1976 Aug 26;295(9):481–485. doi: 10.1056/NEJM197608262950905. [DOI] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M. Pseudomonas aeruginosa: immune status in patients with cystic fibrosis. Infect Immun. 1972 Oct;6(4):628–635. doi: 10.1128/iai.6.4.628-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., MacPherson G. G., Gordon S. Interaction of human monocytes, macrophages, and polymorphonuclear leukocytes with zymosan in vitro. Role of type 3 complement receptors and macrophage-derived complement. J Clin Invest. 1985 Dec;76(6):2368–2376. doi: 10.1172/JCI112249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freimer N. B., Ogmundsdóttir H. M., Blackwell C. C., Sutherland I. W., Graham L., Weir D. M. The role of cell wall carbohydrates in binding of microorganisms to mouse peritoneal exudate macrophages. Acta Pathol Microbiol Scand B. 1978 Apr;86(2):53–57. doi: 10.1111/j.1699-0463.1978.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Glass E., Stewart J., Weir D. M. Presence of bacterial binding 'lectin-like' receptors on phagocytes. Immunology. 1981 Nov;44(3):529–534. [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Gościniak G., Maresz-Babczyszyn J., Grzybek-Hryncewicz K. Phagocytosis and intracellular killing of mucoid and nonmucoid variants of Pseudomonas aeruginosa by polymorphonuclear leukocytes: effect of specific immune sera. Arch Immunol Ther Exp (Warsz) 1984;32(4):467–479. [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S., Holsclaw D. S. Interactions of Pseudomonas aeruginosa with immunoglobulins and complement in sputum. Infect Immun. 1976 Jul;14(1):114–117. doi: 10.1128/iai.14.1.114-117.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hatch G. E., Spock A., Gardner D. E., Menzel D. B. Differences between particulate and peptide stimuli on activation of oxidant production in alveolar macrophages. Chest. 1980 Feb;77(2 Suppl):267–269. doi: 10.1378/chest.77.2.267. [DOI] [PubMed] [Google Scholar]
- Helmke R. J., Boyd R. L., German V. F., Mangos J. A. From growth factor dependence to growth factor responsiveness: the genesis of an alveolar macrophage cell line. In Vitro Cell Dev Biol. 1987 Aug;23(8):567–574. doi: 10.1007/BF02620974. [DOI] [PubMed] [Google Scholar]
- Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis. Relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Aug;82(4):551–558. [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Aeration selects for mucoid phenotype of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):986–990. doi: 10.1128/jcm.24.6.986-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Phosphorylcholine stimulates capsule formation of phosphate-limited mucoid Pseudomonas aeruginosa. Infect Immun. 1988 Apr;56(4):864–873. doi: 10.1128/iai.56.4.864-873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn D. B., Brestel E. P., Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987 Aug;55(8):1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Clawson C. C., Quie P. G., Peterson P. K. Phagocytosis by polymorphonuclear leukocytes of Staphylococcus aureus and Pseudomonas aeruginosa adherent to plastic, agar, or glass. J Immunol Methods. 1983 Sep 30;63(1):103–114. doi: 10.1016/0022-1759(83)90213-2. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meshulam T., Verbrugh H. A., Verhoef J. Opsonization and phagocytosis of mucoid and non-mucoid Pseudomonas aeruginosa strains. Eur J Clin Microbiol. 1982 Apr;1(2):112–117. doi: 10.1007/BF02014202. [DOI] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Chakrabarty A. M. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect Immun. 1982 Aug;37(2):662–669. doi: 10.1128/iai.37.2.662-669.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A. M., Weir D. M. Inhibition of bacterial binding to mouse macrophages by Pseudomonas alginate. J Clin Lab Immunol. 1983 Apr;10(4):221–224. [PubMed] [Google Scholar]
- Pantazis C. G., Kniker W. T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979 Aug;26(2):155–170. [PubMed] [Google Scholar]
- Parod R. J., Brain J. D. Immune opsonin-independent phagocytosis by pulmonary macrophages. J Immunol. 1986 Mar 15;136(6):2041–2047. [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Wood R. E., Robinson R. A., Levine A. S. Use of a Pseudomonas Aeruginosa vaccine in pateints with acute leukemia and cystic fibrosis. Am J Med. 1975 May;58(5):629–636. doi: 10.1016/0002-9343(75)90498-2. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Kazmierowski J. A., Newball H. H. Specificity of opsonic antibodies to enhance phagocytosis of Pseudomonas aeruginosa by human alveolar macrophages. J Clin Invest. 1975 Aug;56(2):376–385. doi: 10.1172/JCI108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Ruhen R. W., Holt P. G., Papadimitriou J. M. Antiphagocytic effect of Pseudomonas aeruginosa exopolysaccharide. J Clin Pathol. 1980 Dec;33(12):1221–1222. doi: 10.1136/jcp.33.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S., Varma R. A simple method for the detection and quantitative determination of hexuronic acids and pentoses. Anal Biochem. 1974 Jan;57(1):268–273. doi: 10.1016/0003-2697(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Yanai M., Quie P. G. Chemiluminescence by polymorphonuclear leukocytes adhering to surfaces. Infect Immun. 1981 Jun;32(3):1181–1186. doi: 10.1128/iai.32.3.1181-1186.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


