Abstract
Background and purpose:
There are limited options for the treatment of neuropathic pain. Endocannabinoids, such as anandamide and 2-arachidonoyl glycerol (2-AG), are promising pain modulators and there is recent evidence of interactions between anandamide and 2-AG biosynthesis and metabolism. It has been clearly demonstrated that 2-AG degradation is mainly catalysed not only by monoacylglycerol lipase (MGL) but also by a fatty acid amide hydrolase (FAAH). Inhibitors specifically targeting these two enzymes have also been described: URB602 and URB597, respectively. However, the anti-nociceptive effects of the combination of peripherally injected 2-AG, URB602 and URB597 in a neuropathic pain model have not yet been determined. This was performed in the presence or absence of cannabinoid CB1 (AM251) and CB2 (AM630) receptor antagonists.
Experimental approach:
Mechanical allodynia and thermal hyperalgesia were evaluated in 213 male Wistar rats allocated to 32 different groups. Drugs were injected subcutaneously in the dorsal surface of the hind paw (50 μL) 15 min before pain tests.
Key results:
2-AG, URB602 and URB597 significantly decreased mechanical allodynia and thermal hyperalgesia with ED50 of 1.6±1.5 and 127±83 μg for 2-AG and URB602, respectively. These effects were mediated locally and were mostly inhibited by the two cannabinoid antagonists.
Conclusions and implications:
The combination of the three compounds did not produce any greater anti-allodynic or anti-hyperalgesic effects, suggesting that FAAH inhibition could reduce or limit the anti-nociceptive effects of 2-AG. Peripheral administration of endocannabinoids or MGL/FAAH inhibitors is a promising analgesic approach requiring further investigation.
Keywords: 2-arachidonoyl glycerol, URB602, URB597, endocannabinoids, cannabinoid receptors, monoacylglycerol lipase, fatty acid amide hydrolase, neuropathic pain
Introduction
Neuropathic pain is a complex disorder resulting from nerve injury or insult to the peripheral or CNS. Unfortunately, it is still considered to be relatively refractory to most of the current analgesics, including opioids, emphasizing the importance of identifying novel pharmacological agents for its treatment. Recent treatment strategies have suggested that cannabinoids may be more effective against chronic than acute pain (Beaulieu and Ware, 2007).
There is accumulating evidence supporting the suggestion that the endogenous cannabinoid system, mainly comprising anandamide and 2-arachidonoyl glycerol (2-AG), may be involved in pain modulation via the activation of two distinct G-protein-coupled receptors, CB1 and CB2 (Pertwee, 2006; Di Marzo, 2008). The cannabinoid CB1 receptor is primarily expressed at high levels in several brain areas and throughout the CNS, including the spinal cord, in neurons of the dorsal root ganglia and also in peripheral tissues (Walczak et al., 2005, 2006; Agarwal et al., 2007; Lever and Rice, 2007). By contrast, cannabinoid CB2 receptor expression seems to be found predominantly, but not exclusively, in peripheral tissues with immune functions. It has also recently been found in the brain, on dorsal root ganglia, in the lumbar spinal cord, on sensory neurons, on microglia and in peripheral tissues (for review see Jhaveri et al., 2007b).
Recent studies have demonstrated the anti-nociceptive efficacy of cannabinoids in several animal models of inflammatory and neuropathic pain (for review see Walker and Hohmann, 2005). Moreover, it has been suggested that cannabinoids can exert their anti-nociceptive action through peripheral mechanisms involving CB1 and CB2 receptors (Sokal et al., 2003; Elmes et al., 2004; Jhaveri et al., 2006; Gutierrez et al., 2007).
In neuropathic pain conditions, several studies have shown that both CB1 (Agarwal et al., 2007) and CB2 (for review see Guindon and Hohmann, 2008) receptors are involved in cannabinoid-induced anti-nociception at peripheral sites. Furthermore, we have already shown that local administration of anandamide into the hind paw reversed mechanical allodynia and thermal hyperalgesia by a mechanism involving only CB1 receptors (Guindon and Beaulieu, 2006).
There is evidence that 2-AG acts as an endogenous ligand, as a full agonist, for both CB1 and CB2 cannabinoid receptors (Mechoulam et al., 1995; Sugiura and Waku, 2000), with no direct binding to the TRPV1 receptor (Pertwee, 2006). However, it seems to act mostly as a potent and full-efficacy agonist at the cannabinoid CB2 receptor (Gonsiorek et al., 2000; Guindon et al., 2007). 2-AG is mainly hydrolysed into arachidonic acid and glycerol by the enzyme monoacylglycerol lipase (MGL) (Goparaju et al., 1999; Dinh et al., 2004). However, evidence suggests that 2-AG might also be metabolized by another enzyme, fatty acid amide hydrolase (FAAH), known to be responsible for the degradation of anandamide (Bisogno et al., 2005), and by two other previously uncharacterized serine hydrolases ABHD6 and ABHD12 (Blankman et al., 2007). A cautious interpretation is that both FAAH and MGL participate in 2-AG inactivation to an extent depending on the tissue or cell type (Di Marzo and Petrosino, 2007). FAAH inhibitors have been described, such as URB597 (Kathuria et al., 2003), and FAAH knockout mice have been developed (Cravatt et al., 2001). More recently, as MGL knockout mice have not yet been described to investigate the precise role of these enzymes in pain modulation, MGL inhibitors, such as URB602 (Hohmann et al., 2005) have been developed.
However, Vandevoorde et al. (2007) and Muccioli et al. (2007) have recently questioned the selectivity of URB602 for MGL. In their study, in vitro URB602 was as effective at inhibiting MGL as FAAH. Nevertheless, recent data using both a purified recombinant MGL and intact brain neurons unequivocally showed that URB602 successfully inhibits MGL and blocks 2-AG hydrolysis in brain slices (King et al., 2007).
Therefore, this study was designed to investigate the peripherally mediated anti-nociceptive effects of 2-AG, URB602 and URB597, after intraplantar injection, in a model of neuropathic pain. The mechanism by which the three compounds might produce their anti-nociceptive effect was also investigated by using specific antagonists for the cannabinoid CB1 and CB2 receptors.
Methods
Animals
The research protocol was approved by the Animal Ethics Committee of the Université de Montréal, and all procedures related to the use of animals conformed to the guidelines of the Canadian Council for Animal Care. A total of 213 adult male Wistar rats (Charles River, St-Constant, Quebec, Canada) weighing 180–220 g, at the time of testing, were housed, two per cage, in standard plastic cages with sawdust bedding in a climate-controlled room, under a 12-h light/dark cycle. They were allowed free access to food pellets and water.
Drug administration
2-AG was purchased from Sigma (St Louis, MO, USA) in acetonitrile solution and further diluted in normal saline (0.9% NaCl in water). AM251 and AM630 are selective antagonists for CB1 and CB2 receptors, respectively, and were purchased from Tocris (Ellisville, MO, USA); they were dissolved in normal saline containing 8 and 2.5% dimethyl sulphoxide (DMSO), respectively. URB602 and URB597 were purchased from Cayman Chemical (Ann Arbor, MI, USA) and dissolved in normal saline containing between 1 and 10% DMSO for the experimental doses. Preliminary experiments with our model of neuropathic pain in rats (n=4–6) have shown that there was no difference in nociceptive responses between the effects of 0.9% NaCl and 0.9% NaCl solution with 8% DMSO (Guindon and Beaulieu, 2006). 2-AG, URB602 and URB597 were stored at −20 °C, protected from light and were prepared freshly on the day of the experiment.
Neuropathic pain model
The partial sciatic nerve ligation (PNL) model was used in this study to achieve unilateral hindlimb neuropathy (Seltzer et al., 1990). Briefly, rats were anaesthetized using isoflurane (induction, 3%; maintenance, 1–2%). The left sciatic nerve was isolated under aseptic conditions and exposed at high thigh level and a one-third to a one-half of the dorsal thickness of the nerve was then tightly ligated using an 8-0 monofilament nylon suture. After surgery, the wound was closed using 5-0 absorbable sutures for muscles and two wound clips for the skin; the rats were allowed 9 days to recover and their posture and behaviour were closely monitored for 48 h. From day 10, rats were tested to assess the development of mechanical allodynia and thermal hyperalgesia. On the 11th day, drugs were administered 15 min prior to nociceptive testing.
Sensory testing
During sensory testing, animals were placed in elevated Plexiglas boxes (21 × 17 × 14 cm) with a 0.7 cm diameter mesh grid floor for mechanical allodynia testing and a dry glass floor for thermal hyperalgesia testing. Rats were allowed to acclimatize for 15 min or until exploratory behaviour ceased before behavioural testing. Effect of drugs with short half-lives, such as 2-AG, may be influenced by the testing order. To minimize this effect, the sequence of testing was alternated between mechanical allodynia and thermal hyperalgesia. The time to complete one battery of tests was approximately 40 min.
Mechanical allodynia
Mechanical sensitivity of the ipsilateral and contralateral hindpaws was assessed using von Frey hairs (Senselab aesthesiometer, Somedic, Sweden). Innocuous mechanical punctuate stimuli were delivered to the plantar surface of the hind paw with a series of individual von Frey filaments of ascending forces (with a range comprised between 0.63 and 300 mN). For each filament, the stimulus was repeated five times with an interval of 1–2 s between each trial. The threshold was determined as the lower force that evoked a withdrawal response to one of the five stimuli (Tal and Bennett, 1994).
Thermal hyperalgesia
Thermal sensitivity of the ipsilateral and contralateral hind paws was assessed using an infrared noxious heat stimulus of increasing temperature range, from non-noxious to noxious range (Plantar test, Ugo Basile, Italy). Thermal punctuate stimuli were delivered to the plantar surface of the hind paw with a focused beam of radiant heat, and the withdrawal latency time was recorded. Results of each test are expressed as the mean of three withdrawal latencies in seconds (s). Three minutes was allowed between each test (Hargreaves et al., 1988).
Experimental protocols
The experiments were conducted in a randomized and blinded manner by the same experimenter. Mechanical allodynia and thermal hyperalgesia were evaluated the day before PNL and on the 10th day after PNL for the ipsilateral and contralateral paws to confirm that rats were neuropathic.
Dose–response curves for 2-AG (0.001–0.01–0.1–1–3–10–30–100 μg; n=4–6 per group) and URB602 (0.1–1–10–100–1000 μg; n=4–6 per group) for mechanical allodynia and thermal hyperalgesia were determined.
On the 11th day, rats were allocated to 32 different groups: (1) a control group for each set (five) of experiments (NaCl 0.9%); (2) 2-AG (10 μg); (3) AM251; (4) AM251+2-AG; (5) AM630; (6) AM630+2-AG; (7) URB602 (300 μg); (8) AM251+URB602; (9) AM630+URB602; (10) URB597 (25 μg); (11) AM251+URB597; (12) AM630+URB597; (13) 2-AG (ED50); (14) URB602 (ED50); (15) 2-AG+URB602; (16) 2-AG+URB597; (17) 2-AG+URB602+URB597. 2-AG, URB602, URB597 (25 μg; Jhaveri et al., 2006), AM251 (80 μg; Malan et al., 2001) and AM630 (25 μg; Malan et al., 2001) were all dissolved in the same total volume of 50 μL and administered subcutaneously (s.c.) in the dorsal surface of the operated left hind paw, 15 min before pain tests (n=6 per group).
To exclude any possible systemic effect of the drugs, NaCl 0.9%, 2-AG, URB602 and URB597 were also given subcutaneously on the dorsal surface of the contralateral side (right hind paw) (n=6 per group).
Statistical analysis
Mechanical threshold and withdrawal latency for each treatment group are expressed as mean±s.e.mean. Assessment of the neuropathy, comparisons between rats receiving NaCl 0.9%, AM251 or AM630 and analgesic effects of 2-AG, URB602, URB597 and their combination with regard to the absence or presence of antagonists (AM251 or AM630) were all assessed separately for significance using an ANOVA adapted for factorial experimental design (Winer, 1971; Draper and Smith, 1998). Each of the analyses mentioned above was assessed for mechanical allodynia and thermal hyperalgesia of the ipsilateral side. The contralateral side was analysed separately, still using an ANOVA adapted for factorial experimental design. The dose–response curves for 2-AG and URB602 were determined using ALLFIT software (De Léan et al., 1978). The critical level of significance was set at 5% (P<0.05).
Results
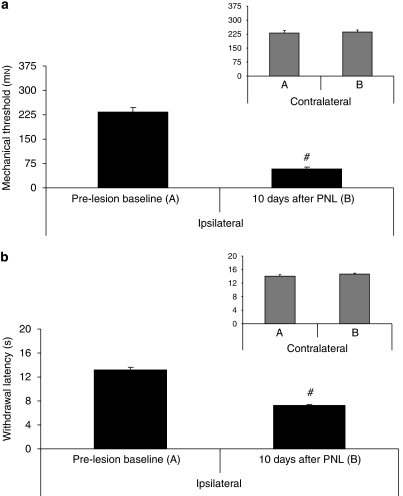
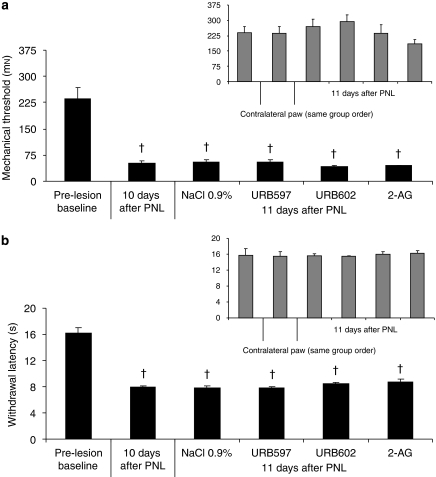
Animals showed signs of neuropathy 10 days after PNL, as a statistically significant difference was present, in comparison with pre-surgery values, for mechanical allodynia (F(1, 94)=142.65, P<0.001) (Figure 1a) and thermal hyperalgesia (F(1, 94)=144.45, P<0.001) (Figure 1b).
Figure 1.
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) before surgery (baseline) and 10 days after surgery consisting of partial sciatic nerve ligation (PNL). #P<0.001 for day 10 vs day 0. Data are expressed as mean±s.e.mean (n=4–6 per group). Contralateral data are presented in the right inset of the figure.
Mechanical allodynia
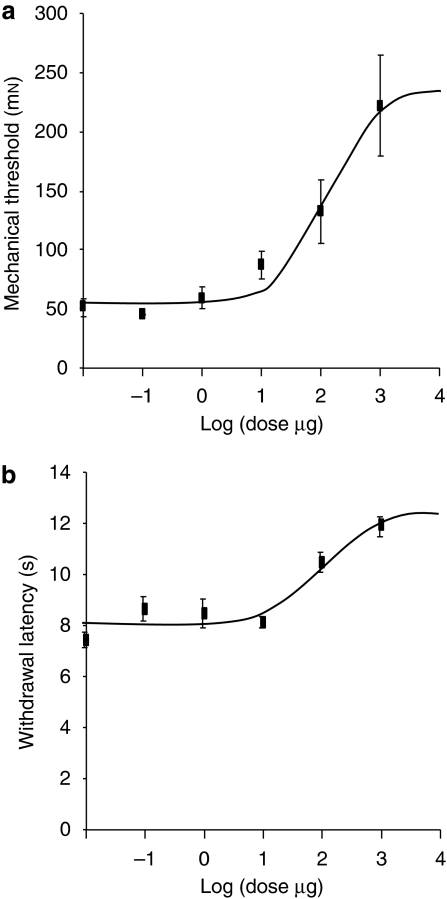
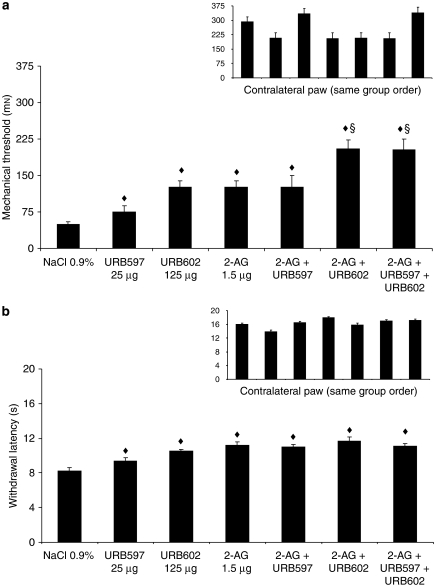
2-AG and URB602 produced a dose-dependent anti-allodynic effect with ED50 of 1.6±1.5 and 127±83 μg, respectively (Figures 2a and 3a). However, a light local intolerance was observed following intraplantar injection of URB602 (1000 μg in 50 μL). This was probably an irritation, so we avoided using this dose. URB597, URB602, 2-AG and their combination produced a significant anti-allodynic effect, when compared with the control group (F(1, 35)=15.68, P<0.001) (Figure 4a). The combination of 2-AG with URB602 or with URB602+URB597 was significantly different when compared with URB597, URB602, 2-AG or 2-AG+URB597 (F(1, 35)=12.93, P<0.005) (Figure 4a), revealing a superior anti-nociceptive effect of those combinations.
Figure 2.
Dose–response curve for 2-arachidonoyl glycerol (2-AG) for mechanical allodynia using von Frey hair (a) and for thermal hyperalgesia using infrared noxious heat stimulus (b). Data are expressed as mean±s.e.mean (n=4–6 per group).
Figure 3.
Dose–response curve for URB602 for mechanical allodynia using von Frey hair (a) and for thermal hyperalgesia using infrared noxious heat stimulus (b). Data are expressed as mean±s.e.mean (n=4–6 per group).
Figure 4.
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) 11 days after surgery for the ipsilateral and contralateral sides (inset). Rats received NaCl 0.9%, URB597 (25 μg), URB602 (125 μg), 2-AG (2-arachidonoyl glycerol; 1.5 μg) or their combination. Data are expressed as mean±s.e.mean (n=6 per group). ♦P<0.001 vs NaCl 0.9%; §P<0.005 vs 2-AG, URB602, URB597 or 2-AG+URB597.
von Frey values obtained in animals after PNL were not affected by treatment with NaCl 0.9%, AM251 or AM630 (F(1, 16)=0.01) (Table 1). Furthermore, values in the control group were not different from values obtained with both cannabinoid antagonists (F(1, 16)=0.01). Finally, results in the AM251 group were not different from those found in the AM630 group (F(1, 16)=0.02) (Table 1).
Table 1.
Mechanical allodynia using von Frey and thermal hyperalgesia using infrared noxious heat stimulus before, 10 and 11 days after surgery
|
Mechanical threshold (mN) |
Withdrawl latency (s) |
|||
|---|---|---|---|---|
| Mean | ±s.e.mean | Mean | ±s.e.mean | |
| Pre-lesion baseline | 301 | 90.8 | 11.4 | 1.7 |
| 10 days after PNL | 50.9 | 11.3 | 6.9 | 0.6 |
| 11 days after PNL-NaCl | 49.2 | 1.1 | 6.6 | 0.6 |
| 11 days after PNL-AM251 | 53.2 | 7.5 | 7.4 | 0.6 |
| 11 days after PNL-AM630 | 45.5 | 0.3 | 7.1 | 0.8 |
Abbreviation: PNL, partial sciatic nerve ligation.
On the 11th day, rats received NaCl 0.9%, AM251 (cannabinoid CB1 antagonist; 80 μg) and AM630 (cannabinoid CB2 antagonist; 25 μg) on the ipsilateral side. Data are expressed as mean±s.e. mean (n=4–6 per group).
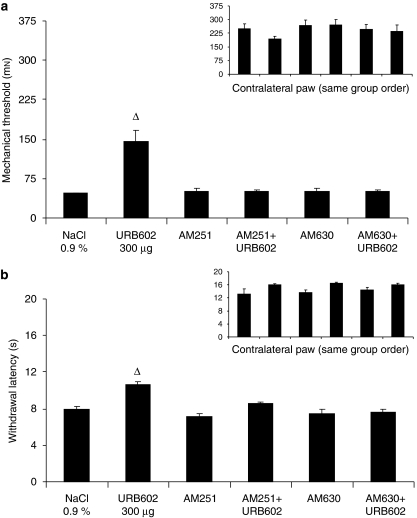
In the absence of cannabinoid antagonists, 2-AG, URB602 and URB597 produced significant anti-allodynic effects, when compared with control group (F(1, 30)=80.41, 52.57 and 4.48), with values returning to pre-surgical levels (Figures 5a, 6a and 7a).
Figure 5.
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) 11 days after surgery for the ipsilateral and contralateral sides (inset). On this day, rats received NaCl 0.9% or 2-AG (2-arachidonoyl glycerol; 10 μg), in the absence or presence of antagonists: AM251 (80 μg)/AM630 (25 μg). Data are expressed as mean±s.e.mean (n=6 per group). *P<0.001 vs NaCl 0.9%.
Figure 6.
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) 11 days after surgery for the ipsilateral and contralateral sides (inset). On this day, rats received NaCl 0.9% or URB602 (300 μg), in the absence or presence of antagonists: AM251 (80 μg)/AM630 (25 μg). Data are expressed as mean±s.e.mean (n=6 per group). ▵P<0.001 vs NaCl 0.9%.
Figure 7.
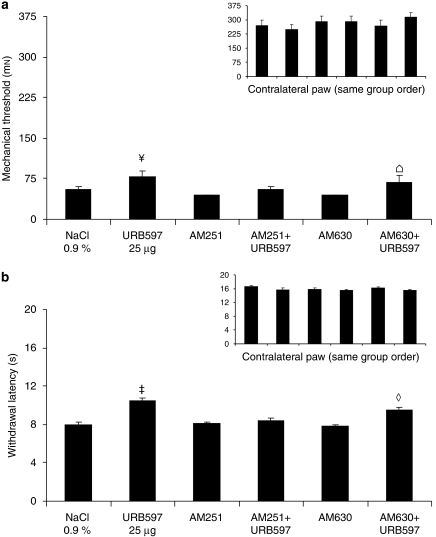
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) 11 days after surgery for the ipsilateral and contralateral sides (inset). On this day, rats received NaCl 0.9% or URB597 (25 μg), in the absence or presence of antagonists: AM251 (80 μg)/AM630 (25 μg). Data are expressed as mean±s.e.mean (n=6 per group). ‡P<0.001 vs NaCl 0.9%; ¥P<0.05 vs NaCl 0.9%;  P<0.05 AM630+URB597 vs AM630; ⋄P<0.001 AM630+URB597 vs AM630.
P<0.05 AM630+URB597 vs AM630; ⋄P<0.001 AM630+URB597 vs AM630.
In the presence of AM251 and AM630, the anti-allodynic effects of 2-AG and URB602 were inhibited, as there was no statistical difference for the comparison between CB1 and CB2 antagonists alone or combined with 2-AG (F(1, 30)=0.61 and 0.14, respectively) (Figure 5a) and with URB602 (F(1, 30)=0.01 and 0.01, respectively) (Figure 6a). The combination of URB597+AM251 (CB1 antagonist) did not produce any anti-allodynic effects, when compared with AM251 alone (F(1, 30)=0.95). By contrast, the combination of URB597+AM630 (CB2 antagonist) did produce significant anti-allodynic effects, when compared with AM630 alone (F(1, 30)=5.32) (Figure 7a). Finally, there was no difference between all the groups tested on the contralateral side (Figures 4a, 5a, 6a and 7a, insets).
When the drugs were given on the contralateral side to rats after PNL (F(1, 30)=265.32, P<0.001), there was no statistical difference between days 10 and 11 (that is, before and after local drug administration) (F(1, 30)=0.01) on the ipsilateral (tested) side (Figure 8a). On the contralateral side, there was no difference between the day before the surgery (pre-lesion) compared with the days after surgery (days 10 and 11) (F(1, 30)=0.49), and no difference was observed between days 10 and 11 (F(1, 30)=0.77) (Figure 8a, inset).
Figure 8.
Mechanical allodynia using von Frey hair (a) and thermal hyperalgesia using infrared noxious heat stimulus (b) before, 10 and 11 days after surgery partial sciatic nerve ligation (PNL), for the ipsilateral and contralateral sides (inset). On the 11th day, rats received NaCl 0.9%, 2-AG (2-arachidonoyl glycerol; 100 μg), URB602 (1000 μg) and URB597 (25 μg) on the contralateral side. Data are expressed as mean±s.e.mean (n=6 per group). †P<0.001 vs baseline.
Thermal hyperalgesia
2-AG and URB602 also produced a dose-dependent anti-hyperalgesic effect with ED50 of 0.20±0.19 and 86±52 μg, respectively (Figures 2b and 3b). 2-AG, URB602, URB597 and their combination produced a significant anti-hyperalgesic effect, when compared with the control group (F(1, 35)=38.74, P<0.001) (Figure 4b). The combination of 2-AG with URB602 or with URB602+URB597 was not significantly different when compared with 2-AG, URB602, URB597 or 2-AG+URB597 (F(1, 35)=2.67), showing that there was no greater anti-nociceptive effect of the combinations.
Responses to the noxious heat stimulus obtained after PNL were not different (F(1, 16)=0.02) from those measured when rats received NaCl 0.9%, AM251 or AM630. Furthermore, values in the control group were not different from values obtained with both cannabinoid antagonists (F(1, 16)=0.35). Finally, results in the AM251 group were not different from those found in the AM630 group (F(1, 16)=0.05) (Table 1).
In the absence of cannabinoid antagonists, 2-AG, URB602 and URB597 produced significant anti-hyperalgesic effects, when compared with control group (F(1, 30)=26.67, 32.58 and 31.59, respectively, P<0.001) with values returning to pre-surgical levels (Figures 5b, 6b and 7b).
In the presence of AM251 and AM630, the anti-hyperalgesic effects of 2-AG and URB602 were inhibited, as there was no statistical difference for the comparison between CB1 and CB2 antagonists alone or combined with 2-AG (F(1, 30)=1.45) (Figure 5b) and URB602 (F(1, 30)=1.32) (Figure 6b). However, the anti-hyperalgesic effects of URB597 were reversed by the CB1 antagonist AM251 (F(1, 30)=0.37), but were not inhibited by the CB2 antagonist AM630 (F(1, 30)=15.26) (Figure 7b). Finally, there was no difference between all the groups tested on the contralateral side (Figures 4b, 5b, 6b and 7b, insets).
When the drugs were given on the contralateral side to rats after PNL (F(1, 30)=254.56, P<0.001), there was no statistical difference between days 10 and 11 (F(1, 30)=0.42) on the ipsilateral side (Figure 8b). On the contralateral side, there was no difference between the day before the surgery (pre-lesion) compared with the days after surgery (days 10 and 11) (F(1, 30)=0.01), and no difference was observed between days 10 day 11 (F(1, 30)=0.30) (Figure 8b, inset).
Discussion
In this study, we have demonstrated that local administration of 2-AG, an endocannabinoid, URB602, a MGL inhibitor and URB597, a selective FAAH inhibitor, induced anti-allodynic and anti-hyperalgesic effects in a rat model of neuropathic pain. These anti-nociceptive effects were locally, rather than systemically, mediated, as 2-AG, URB602 and URB597 injected in the contralateral paw did not produce anti-nociceptive effects at doses higher than those used in the ipsilateral paw. Furthermore, these effects were likely to be mediated by cannabinoid CB1 and CB2 receptors, as anti-nociceptive effects of intraplantar 2-AG and URB602 were prevented by the selective CB1 and CB2 receptor antagonists, AM251 and AM630, respectively. However, the anti-nociceptive effects of intraplantar URB597 were not prevented by AM630, confirming the fact that URB597 produces its effects via direct or indirect activation of CB1 receptors. Thus, these findings suggest that the activation of peripheral CB1 and CB2 receptors by endocannabinoids or with MGL and FAAH inhibitors are sufficient to produce anti-allodynic and anti-hyperalgesic effects.
We have shown that 2-AG produced dose-dependent anti-nociceptive effects and significantly decreased mechanical allodynia and thermal hyperalgesia with ED50 in the microgram range, whereas a recent study from our group showed that anandamide was also anti-nociceptive but with doses in the nanogram range (Guindon and Beaulieu, 2006). In fact, anandamide is a partial or full agonist of CB1 receptors: it mainly mediates its anti-nociceptive effects through the activation of CB1 receptors and its pro-nociceptive effects through TRPV1 receptors (Maccarrone et al., 2008). Although it also binds CB2 receptors, it has very low efficacy and may act as an antagonist (Gonsiorek et al., 2000). In contrast, 2-AG acts as an agonist for both CB1 and CB2 receptors to modulate pain transmission in a model of neuropathic pain (Saario and Laitinen 2007).
MGL inhibitors such as URB602 (Hohmann et al., 2005) and selective FAAH inhibitors such as URB597 (Kathuria et al., 2003; Jayamanne et al., 2006) have been described. URB602 potentitates endocannabinoid-dependent stress-induced analgesia following local administration into either the periaqueductal grey or lumbar dorsal horn (Hohmann et al., 2005; Suplita et al., 2006). Accordingly, ultrastructural studies suggest that MGL is localized predominantly, if not exclusively on axon terminals (Gulyas et al., 2004). Thus, peripherally injected MGL inhibitors, as demonstrated in this study, could offer significant therapeutic potential for the treatment of neuropathic pain.
Although URB602 is an inhibitor of MGL, it has low potency (Hohmann et al., 2005). In addition, Begg et al. (2005) have recently shown evidence for cannabinoid receptors beyond CB1 and CB2. Thus, 2-AG anti-nociceptive action and degradation appear to be complex, to involve multiple enzymes and could vary between cell types. In fact, Muccioli et al. (2007) have reported the existence of a novel, pharmacologically distinct MGL that opens promising therapeutic avenues. However, our current knowledge of the roles of 2-AG in pain modulation is still limited, due to the lack of mutant MGL−/− mice that are incapable of synthesizing or inactivating 2-AG. Nevertheless, we then wanted to evaluate whether 2-AG degradation could be affected both by MGL and by FAAH inhibitors. In fact, the addition of URB597, either with 2-AG or with 2-AG and URB602, failed to produce a significant additive anti-nociceptive effect. These results are in agreement with a recent publication by Maccarrone et al (2008), suggesting a ‘reconceptualization' of the endocannabinoid system. Indeed, they proposed that anandamide and 2-AG may cooperate to modulate synaptic transmission. In fact, they have demonstrated that the elevation of anandamide concentrations by either pharmacological or genetic inhibition of FAAH can interfere with 2-AG levels, metabolism and physiological effects through the activation of TRPV1 receptors in the striatum (Maccarrone et al., 2008). This interaction could also be present in the periphery, as nociceptive primary sensory neurons co-express CB1 and TRPV1 receptors to a very high degree (Ahluwalia et al., 2000). However, it may not be adequate to apply this new concept, based on findings in the CNS, to events in the periphery.
Several recent studies have reported that both peripheral CB1 (Agarwal et al., 2007) and CB2 (for review see Guindon and Hohmann, 2008) receptors are involved in anti-allodynic and anti-hyperalgesic effects of locally injected cannabinoids after nerve injury. In peripheral tissues, the expression of CB1 and CB2 receptors in rats and mice has been well described not only at basal level, but was also upregulated in the paw, DRG and ipsilateral spinal cord of rats in a new model of neuropathic pain, targeting the saphenous nerve (Walczak et al., 2005, 2006). Similarly, Mitrirattanakul et al. (2006) have reported that CB1 receptors are upregulated in DRG following the development of neuropathic pain, thereby providing hope for the use of compounds targeting the peripheral endocannabinoid system. Furthermore, studies of knockout mice have confirmed that CB1 and CB2 receptors are involved in cannabinoid-induced analgesia (Ledent et al., 1999; Zimmer et al., 1999; Ibrahim et al., 2006), but have not revealed their precise site of action. To resolve this question, Agarwal et al. (2007) generated transgenic mice lacking CB1 receptors in nociceptive neurons localized in the peripheral nervous system of mice, preserving expression in spinal neurons, the brain and all other organs. They showed that CB1 receptors on nociceptors, and not those within the CNS, constitute an important target for mediating cannabinoid analgesia (Agarwal et al., 2007). However, one cannot rule out compensatory effects in transgenic mouse models. Furthermore, previous studies have reported that endocannabinoids, such as anandamide and 2-AG, are present in peripheral tissues (Beaulieu et al., 2000; Jhaveri et al., 2006; Mitrirattanakul et al., 2006). Elevation of endocannabinoids levels has been demonstrated in paw tissues following tissue injury (Calignano et al., 1998; Oka et al., 2005; Mitrirattanakul et al., 2006), in the spinal cord during L5–L6 spinal nerve ligation (Jhaveri et al., 2006) and chronic constriction injury of the sciatic nerve (Petrosino et al., 2007). Therefore, endocannabinoid levels in neuropathic pain conditions are consistently reported to be increased at many sites involved in the nociceptive processing (Jhaveri et al., 2007a). Indeed, Jhaveri et al. (2006) have recently demonstrated that intraplantar injection of URB597 suppressed the mechanically evoked responses of wide dynamic-range spinal neurons in sham-operated rats and increased anandamide and 2-AG levels. However, a higher dose of intraplantar URB597 was required to attenuate evoked responses of spinal neurons in nerve-ligated rats, but surprisingly failed to alter hind paw levels of endocannabinoids. The authors suggested that these levels may have been affected by other metabolic pathways, such as cyclooxygenase-2 (COX-2), or alterations of peripheral FAAH activity (Jhaveri et al., 2006). Measurement of endocannabinoid levels in peripheral paw tissue was not performed in the present study. The absence of such measures is a weakness and to that extent, the results presented herein do not contribute to the debate regarding the specificity of URB602 for MGL inhibition. It is, however, interesting to note that in a recent publication, concerns were raised about the correlation between the amounts of endocannabinoids measured in tissues and extracellular, and hence CB receptor-active, levels of endocannabinoids (Di Marzo, 2008). Endocannabinoids might be present in high concentrations to inhibit pain transmission by acting on CB1 and CB2 receptors, which have also been found to be upregulated in several regions of the pain pathway. Therefore, the anti-nociceptive effects of 2-AG may be mediated by a direct action on cannabinoid CB1 and CB2 receptors located in the injected paw.
Other mechanisms to explain the peripheral anti-hyperalgesic action of cannabinoids may be linked to the anti-inflammatory effects of these compounds in peripheral tissues; CB1 and CB2 receptor agonists could act locally at the level of the peripheral axon of the primary afferent neuron and indirectly inhibit transmission in nociceptive afferents by reducing the inflammatory component of tissue injury. In fact, recent studies have reported that cannabinoids can reduce the production and release of pro-inflammatory signalling molecules, such as tumour necrosis factor-α, nitric oxide and interleukin-1 (IL-1), and enhance the release of anti-inflammatory cytokines like the IL-1 receptor antagonist, IL-4 and IL-10 (Walter and Stella, 2004). Moreover, another hypothesis is that the activation of CB2 cannabinoid receptors may stimulate the local release of the endogenous opioid endorphin from cells such as keratinocytes (Ibrahim et al., 2005). These authors have postulated the release of β-endorphin by AM1241, a CB2 selective agonist, acting on CB2 receptors in skin keratinocytes, as a mechanism to explain the peripheral analgesic actions of this compound. Therefore, endocannabinoids such as anandamide and 2-AG may interact directly or indirectly with cannabinoid receptors on the peripheral endings of cutaneous nociceptives sensory neurons to modulate the transmission of nociceptive signals to the spinal cord.
The anti-nociceptive effects of peripherally administered cannabinoids need to be counterbalanced by the fact that many reports have already demonstrated anti-nociceptive properties of central (cerebral and spinal) administrations of cannabinoid receptor agonists (for review see Hohmann et al., 2005; Lever and Rice, 2007), whereas others have questioned the involvement of CB1 receptors in the analgesia evoked by peripheral administration of cannabinoids.
In conclusion, these findings are the first to demonstrate that peripherally injected 2-AG, URB602, URB597 and their combination decreased pain behaviour in a model of neuropathic pain. This study provides evidence that the activation of cannabinoid CB1 and CB2 mechanisms in the periphery is sufficient to decrease nociception in an animal model of neuropathic pain. Finally, as cannabinoid-based drugs seem to be effective for the treatment of neuropathic pain, this approach is of potential therapeutic importance because it may present a way of delivering the analgesic benefit of cannabinoid compounds while avoiding troublesome psychotropic and other systemic effects.
Acknowledgments
JD is supported by a Groupe de Recherche Universitaire sur le Médicament (GRUM) Pfizer doctoral study grant. JG is supported by a Fonds de la Recherche en Santé du Quebec (FRSQ) postdoctoral fellowship. PB holds an FRSQ clinician-scientist grant.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- CB
cannabinoid
- DMSO
dimethylsulphoxide
- FAAH
fatty acid amide hydrolase
- MGL
monoacylglycerol lipase
Conflict of interest
The authors state no conflict of interest.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Beaulieu P, Bisogno T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, et al. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol. 2000;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Beaulieu P, Ware M. Reassessment of the role of cannabinoids in the management of pain and other symptoms. Curr Opin Anaesthesiol. 2007;20:473–477. doi: 10.1097/ACO.0b013e3282efd175. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Léan A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce. Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Draper NR, Smith H. Applied Regression Analysis. Wiley: New York; 1998. [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naïve rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999;57:417–423. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology. 2006;50:814–823. doi: 10.1016/j.neuropharm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdale. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007a;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Sagar DR, Elmes SJ, Kendall DA, Chapman V. Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain. Mol Neurobiol. 2007b;36:26–35. doi: 10.1007/s12035-007-8007-7. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Rice AS. Cannabinoids and pain. Handb Exp Pharmacol. 2007;177:265–306. doi: 10.1007/978-3-540-33823-9_10. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, et al. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126:102–114. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG, Xu C, Odah E, Cudaback E, Cisneros JA, Lambert DM, et al. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J Neurosci. 2007;27:2883–2889. doi: 10.1523/JNEUROSCI.4830-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, et al. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, et al. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sokal DM, Elmes SJ, Kendall DA, Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45:404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids. 2000;108:89–106. doi: 10.1016/s0009-3084(00)00189-4. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Gutierrez T, Fegley D, Piomelli D, Hohmann AG. Endocannabinoids at the spinal level regulate, but do not mediate, nonopioid stress-induced analgesia. Neuropharmacology. 2006;50:372–379. doi: 10.1016/j.neuropharm.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Saario SM, Laitinen JT. Therapeutic potential of endocannabinoid-hydrolysing enzyme inhibitors. Basic Clin Pharmacol Toxicol. 2007;101:287–293. doi: 10.1111/j.1742-7843.2007.00130.x. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–191. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132:1093–1102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Characterization of the chronic constriction of the saphenous nerve, a model of neuropathic pain in mice showing rapid molecular and electrophysiological changes. J Neurosci Res. 2006;83:1310–1322. doi: 10.1002/jnr.20821. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005;168:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. McGrawHill: New York; 1971. [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]