Abstract
Background and purpose:
There is growing interest in using cannabinoid type 2 (CB2) receptor agonists for the treatment of neuropathic pain. In this report, we describe the pharmacological characteristics of MDA7 (1-[(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl]piperidine), a novel CB2 receptor agonist.
Experimental approach:
We characterized the pharmacological profile of MDA7 by using radioligand-binding assays and in vitro functional assays at human cannabinoid type 1 (CB1) and CB2 receptors. In vitro functional assays were performed at rat CB1 and CB2 receptors. The effects of MDA7 in reversing neuropathic pain were assessed in spinal nerve ligation and paclitaxel-induced neuropathy models in rats.
Key results:
MDA7 exhibited selectivity and agonist affinity at human and rat CB2 receptors. MDA7 treatment attenuated tactile allodynia produced by spinal nerve ligation or by paclitaxel in a dose-related manner. These effects were selectively antagonized by a CB2 receptor antagonist but not by CB1 or opioid receptor antagonists. MDA7 did not affect rat locomotor activity.
Conclusion and implications:
MDA7, a novel selective CB2 agonist, was effective in suppressing neuropathic nociception in two rat models without affecting locomotor behaviour. These results confirm the potential for CB2 agonists in the treatment of neuropathic pain.
Keywords: cannabinoid, CB2, MDA7, neuropathic pain, allodynia, hyperalgesia, cancer, chemotherapy
Introduction
Neuropathic pain can be a debilitating condition characterized by severe, persistent pain that is refractory to traditional analgesics. Neuropathic pain results from several different conditions affecting the peripheral or CNS and affects an estimated 8% of people worldwide (Torrance et al., 2006). The annual U.S. health-care cost attributable to neuropathic pain is almost $40 billion (Turk, 2002). The currently available treatments for neuropathic pain—opioids, gabapentin and amitriptyline—are effective in fewer than 30% of patients (Warms et al., 2002; Davis, 2007; Khaliq et al., 2007; Tassone et al., 2007).
Cannabis extracts, such as Marinol and Sativex, and synthetic derivatives, such as Cannador, have also been tried as treatments for neuropathic pain. However, these have limited efficacy in humans and they contain Δ9-THC, the natural constituent of hemp (Cannabis sativa), which produces adverse psychotropic effects (Karst et al., 2003; Berman et al., 2004; Woolridge et al., 2005; Holdcroft et al., 2006; Abrams et al., 2007; Nurmikko et al., 2007). These cannabis extracts and synthetic derivatives have agonist activity for human cannabinoid type 1 (CB1) receptors and inverse agonist activity at cannabinoid type 2 (CB2) receptors (Govaerts et al., 2004; receptor nomenclature according to Alexander et al., 2008).
Both CB1 and CB2 receptors have been characterized and cloned (Devane et al., 1988; Matsuda et al., 1990; Munro et al., 1993). There is also evidence for the existence of additional pharmacologically identified, non-CB1/CB2 receptors (Overton et al., 2006; Ryberg et al., 2007). CB2 receptors are expressed primarily in the peripheral immune system (Munro et al., 1993; Facci et al., 1995) and to a lesser degree in the CNS (Van Sickle et al., 2005; Onaivi et al., 2006). CB2 receptor expression was found in the brainstem neurons of wild-type mice but was absent in CB2-receptor knockout mice (Van Sickle et al., 2005). Onaivi et al. (2006) reported that CB2 receptors were expressed in the brains of naive mice and that this expression was enhanced in the presence of chronic mild stress. Recently, a pharmacological magnetic resonance imaging study demonstrated that even if CB2 receptors are expressed in the brain, they are not functional under normal physiological conditions (Chin et al., 2008). Human and rat CB1 and CB2 receptors show 97 and 81% amino-acid sequence identity, respectively (Matsuda et al., 1990; Gerard et al., 1991; Reggio, 2003). The CB2 receptor shows 44% identity with the CB1 receptor (Munro et al., 1993).
Several studies have demonstrated that CB2-selective ligands have a significant function in the modulation of pain perception but do not produce the adverse psychoactive effects associated with CB1 receptor activation (Huffman et al., 1996; Huffman, 2005; Salo et al., 2005; Valenzano et al., 2005; Manera et al., 2006; Murineddu et al., 2006; Giblin et al., 2007; Ohta et al., 2007a, 2007b; Page et al., 2007; Yao et al., 2008). However, despite several publications concerning the therapeutic potential of CB2-selective ligands as novel analgesic agents, conflicting results exist with respect to their in vitro and in vivo profiles. AM1241 [(2-iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl-methanone], which has been used as a reference compound to study the effects of CB2 agonists (Ibrahim et al., 2003, 2006; Beltramo et al., 2006), appears to be a weak partial agonist for human CB2 receptors and an inverse agonist for rat CB2 receptors (Bingham et al., 2007). Furthermore, interactions of AM1241 with the opioid system may contribute to its in vivo properties (Ibrahim et al., 2005). Other CB2-selective agonists that have been described include HU-308 (Hanus et al., 1999), GW405833 (L-768,242) [(2,3-dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-morpholin-4-yl-ethyl)-indol-1-yl]-methanone] (Valenzano et al., 2005), Sch35966 (Gonsiorek et al., 2007), GW842166X (2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide) (Giblin et al., 2007), A-796260 ([1-(2-morpholin-4-yl-ethyl)-1H-indol-3-yl]-(2,2,3,3-tetramethylcyclopropyl)-methanone) (Yao et al., 2008), AM1241 (Ibrahim et al., 2003) and PF-03550096 (Kikuchi et al., 2008). A dose of 50 mg kg−1 of HU-308 given i.p. was reported to be effective in reversing the late but not the early phase of formalin-induced peripheral pain in mice and this effect was blocked by the CB2 antagonist SR-144528 (Hanus et al., 1999). GW405833 (L-768,242) exhibited partial agonist activity at human CB2 receptors and no functional data were reported on rat CB2 receptors (Valenzano et al., 2005). The antiallodynic effect of GW405833 was found to be less than that of AM1241 (LaBuda et al., 2005). GW842166X was reported to be effective in the Freund's complete adjuvant model of inflammatory pain (with an oral ED50 of 0.1 mg kg−1) (Giblin et al., 2007). In a spinal nerve ligation neuropathic pain model, doses of up to 30 mg kg−1 of GW842166X i.p. produced an effect comparable to that of 50 mg kg−1 of gabapentin p.o. (Conti et al., 2007). PF-03550096 (Kikuchi et al., 2008) was effective in attenuating visceral hypersensitivity induced by 2,4,6-trinitrobenzene sulphonic acid but was not tested in a neuropathic pain model. Similarly, no in vivo activities were reported for Sch35966 (Gonsiorek et al., 2007). A-796260 (Yao et al., 2008) has been shown to be effective in a sciatic nerve chronic constriction neuropathic pain model. However, doses up to 35 mg kg−1 of A-796260 i.p. were less effective than 6 mg kg−1 of morphine i.p. in reversing allodynia (Yao et al., 2008).
In the studies described here, we tested the efficacy of a novel CB2-selective agonist, MDA7 (1-[(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl]piperidine; Figure 1a), based on a 3,3-disubstituted-2,3-dihydro-1-benzofuran scaffold obtained by a colloidal palladium nanoparticle-catalysed tandem cyclization/cross-coupling reaction (Figure 1b). By using binding and functional assays and in vivo models of neuropathic pain, we demonstrated that MDA7 is a selective agonist for CB2 receptors that suppressed neuropathic nociception.
Figure 1.
(a) Comparison of isatin and benzofuran structures. Ring B from the benzofuran series is superimposable on the six-membered ring formed by the hydrazone and the indolone through an internal hydrogen bond. In this case, both five-membered rings (ring A) from the isatin and benzofuran structures also fit. (b) Summary of synthetic pathway for MDA7 (see Methods for details).
Methods
Synthesis of MDA7
MDA7 was synthesized as shown in Figure 1b. Briefly, 3-hydroxy-4-iodo-benzoic acid was obtained by iodination (NaI, NaOCl, NaOH, MeOH, 80% yield) of meta-hydroxybenzoic acid (Edgar and Falling, 1990). The corresponding methyl benzoate was obtained by esterification (MeOH, H2SO4). The phenol derivative was then coupled with 3-bromo-2-methyl-propene by using potassium carbonate in methylethylketone in 98% yield. The resulting compound was submitted to a palladium-catalysed tandem cyclization/Suzuki-coupling reaction (Szlosek-Pinaud et al., 2007) to afford the corresponding benzofuran in 95% yield. MDA7 was obtained after saponification (97% yield) and coupling with piperidine (71% yield).
Analytical data for MDA7
1-[(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl]piperidine, 1H NMR (CDCl3): δ 1.37 (s, 3H), 1.58–1.69 (m, 6H), 2.8 (d, J=13.5 Hz, 1H), 2.90 (d, J=13.5 Hz, 1H), 3.34 (br s, 2H), 3.68 (br s, 2H), 4.09 (d, J=8.7 Hz, 1H), 4.53 (d, J=8.7 Hz, 1H), 6.75 (d, J=1.2 Hz, 1H), 6.88 (dd, J=1.2 Hz, J=7.5 Hz), 6.94 (d, J=7.5 Hz, 1H), 6.99–7.02 (m, 2H), 7.22–7.24 (m, 3H). 13C NMR (CDCl3): δ 24.56 (CH3), 24.64 (CH2), 25.67 (CH2), 26.56 (CH2), 43.15 (CH2), 46.22 (C), 46.57 (CH2), 48.75 (CH2), 82.28 (CH2), 108.20 (CH), 119.09 (CH), 123.43 (CH), 126.58 (CH), 127.99 (CH), 130.36 (CH), 136.21 (C), 136.75 (C), 137.26 (C), 159.47 (C), 170.19 (C=O). HRMS (ES+) calcd for C22H25NO2 (M+ H+), m/e, 336.1964; found, 336.1958.
In vitro receptor radioligand-binding studies
Human CB1 and CB2 receptor-binding studies
MDA7 was screened in a competitive binding experiment by using membranes of Chinese hamster ovarian cells (CHO-K1 cells) expressing human CB1 receptors at different MDA7 concentrations in duplicate (Mukherjee et al., 2004). The competitive binding experiment was performed in 96-well plates (Masterblock, catalogue number 786201, Greiner Bio-One, San Diego, CA, USA) containing binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.5% protease-free BSA, saponin 10 μg mL−1), recombinant membrane extracts (2 μg protein per well) and 1 nM [3H]SR141716A (5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1H-pyrazole-3-carboxylic acid piperidin-1-ylamide), a selective CB1 receptor antagonist (GE Healthcare, TRK1028, 42 Ci mmol−1, diluted in binding buffer). Nonspecific binding was determined in the presence of 10 μM CP55,940 (5-(1,1-dimethylheptyl)-2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxy-propyl)-cyclohexyl]-phenol) (Tocris Bioscience, Ellisville, MO, USA). The sample was incubated in a final volume of 0.1 mL for 60 min at 25 °C and then filtered on a GF/C UniFilter microplate (Perkin Elmer, Waltham, MA, USA; catalogue number 6005177) presoaked in 0.05% Brij for 2 h at room temperature. Filters were washed six times with 4 mL of cold binding buffer, and bound [3H]SR141716A was determined by liquid scintillation counting. The median IC50 was determined by nonlinear regression by using the one-site competition equation. The inhibition constant (Ki) values were calculated by using the Cheng–Prusoff equation (Ki=IC50/(1+(L/KD)), where L=concentration of radioligand in the assay and KD=affinity of the radioligand for the receptor.
MDA7 was also screened in a competitive binding experiment by using membranes of CHO-K1 cells selectively expressing the human CB2 receptor at different MDA7 concentrations in duplicate (Mukherjee et al., 2004). The competitive binding experiment was performed in 96-well plates (Masterblock) containing binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.5% protease-free BSA), recombinant membrane extracts (0.25 μg protein per well) and 1 nM [3H]CP55,940 (Perkin Elmer, NEX-1051, 161 Ci mmol−1, diluted in binding buffer). Nonspecific binding was determined in the presence of 10 μM CP55,940 (Tocris Bioscience). The sample was incubated in a final volume of 0.1 mL for 60 min at 30 °C and then filtered on a GF/B UniFilter microplate (Perkin Elmer, catalogue number 6005177) presoaked in 0.5% polyethyleneimine for 2 h at room temperature. Filters were washed six times with 4 mL of cold binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.5% protease-free BSA), and bound [3H]CP55,940 was determined by liquid scintillation counting. The IC50 and Ki values were calculated as above.
Rat CB1 and CB2 receptor-binding studies
MDA7 was screened in a competitive binding experiment by using membranes of CHO-K1 cells selectively expressing the rat CB1 receptor at different MDA7 concentrations in duplicate (Mukherjee et al., 2004). The competitive binding experiment was performed in 96-well plates (Masterblock) containing binding buffer (50 mM Tris, pH 7.4, 1 mM EDTA, 0.5% protease-free BSA, 3 mM MgCl2), recombinant membrane extracts (25 μg protein per well) and 1.25 nM [3H]SR141716A (5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1H-pyrazole-3-carboxylic acid piperidin-1-ylamide), a selective CB1 receptor antagonist (GE Healthcare diluted in binding buffer). Nonspecific binding was determined in the presence of 10 μM AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methy l-1H-pyrazole-3-carboxamide) (Tocris Bioscience). The sample was incubated in a final volume of 0.1 mL for 60 min at 25 °C and then filtered on a GF/C UniFilter microplate (Perkin Elmer, catalogue number 6005177) presoaked in 0.05% Brij for 2 h at room temperature. Filters were washed six times with 4 mL of cold binding buffer, and bound [3H]SR141716A was determined by liquid scintillation counting. IC50 was determined by nonlinear regression by using the one-site competition equation. The Ki values were calculated using the Cheng–Prusoff equation.
MDA7 was also screened in a competitive binding experiment using membranes of CHO-K1 cells selectively expressing the rat CB2 receptor at different MDA7 concentrations in duplicate (Mukherjee et al., 2004). The competitive binding experiment was performed in 96-well plates (Masterblock) containing binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.5% protease-free BSA), recombinant membrane extracts (0.25 μg protein per well) and 1 nM [3H]CP55,940 (Perkin Elmer, NEX-1051, 161 Ci mmol−1, diluted in binding buffer). Nonspecific binding was determined in the presence of 10 μM CP55,940 (Tocris Bioscience). The sample was incubated in a final volume of 0.1 mL for 60 min at 30 °C and then filtered on a GF/B UniFilter microplate (Perkin Elmer, catalogue number 6005177) presoaked in 0.5% polyethyleneimine for 2 h at room temperature. Filters were washed six times with 4 mL of cold binding buffer (50 mM Tris, pH 7.4, 2.5 mM EDTA, 0.5% protease-free BSA), and bound [3H]CP55,940 was determined by liquid scintillation counting. IC50 and Ki values were calculated as described previously.
GTPγ[35S] functional assays
Functional activity was evaluated by using a GTPγ[35S] assay in CHO-K1 cell membrane extracts expressing recombinant human or rat CB1 or CB2 receptors. The assay relies on the binding of GTPγ[35S], a radiolabeled nonhydrolyzable GTP analogue, to the G-protein upon binding of an agonist of the G-protein-coupled receptor. In this system, agonists stimulate GTPγ[35S] binding, whereas neutral antagonists have no effect and inverse agonists decrease GTPγ[35S] basal binding.
MDA7 was dissolved in 100% dimethylsulphoxide (DMSO) at a concentration of 10 mM within 4 h of the first testing session (master solution). A pre-dilution for the dose–response curve was performed in 100% DMSO, and this solution was diluted 100-fold in assay buffer to a concentration 2-fold higher than the concentration to be tested. MDA7 was tested for agonist and antagonist activities at eight concentrations in duplicate: 10, 3, 1, 0.3, 0.1, 0.03, 0.01 and 0.001 μM. CP55,940 (Tocris Bioscience) was the reference agonist. For GTPγS, 5 μg of membranes were mixed with guanosine-5'-diphosphate diluted in assay buffer to give 30 μM solution (volume/volume), and incubated for at least 15 min on ice. In parallel, GTPγ[35S] (GE Healthcare, catalogue number SJ1308) was mixed with scintillation beads (PVT-WGA, GE Healthcare, catalogue number RPNQ001), diluted in assay buffer at 50 mg mL−1 (0.5 mg per 10 μL−1) (volume/volume) just before the reaction was started. The following reagents were successively added in the wells of an OptiPlate (Perkin Elmer): 50 μL of ligand or the reference antagonist (AM251, a CB1 receptor-selective antagonist); 20 μL of the membrane/guanosine-5'-diphosphate mix; 10 μL of the reference agonist (CP55,940) at historical EC80 (30 nM); and 20 μL of the GTPγ[35S]:beads mix. The plates were covered with a topseal, shaken on an orbital shaker for 2 min and then incubated for 1 h at room temperature. Then the plates were centrifuged for 10 min at 800 g and counted for 1 min per well with a Perkin Elmer TopCount scintillation counter. Assay reproducibility was monitored by the use of the reference agonist, CP55,940. For replicate determinations, the maximum variability tolerated in the test was ±20% of the average of the replicates. Efficacies (Emax) for CB1 or CB2 receptors were expressed as a percentage relative to the efficacy of CP55,940.
Animals
All animal procedures were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center. Animals were housed three per cage on a 12/12 h light/dark cycle with water and food pellets available ad libitum. Adult male Sprague–Dawley (Harlan Sprague Dawley, Indianapolis, IN, USA) rats weighing 120–150 g were used and all experiments were performed during the light cycle.
Spinal nerve (L5/6) ligation model of neuropathic pain
All surgical procedures were performed under deep isoflurane anaesthesia in 100% O2. The spinal nerve ligation was performed as described previously (Kim and Chung, 1992). Briefly, a midline incision was made above the lumbar spine to expose the left L6 transverse process. The process was then removed, the left L5 and L6 spinal nerves were isolated and both nerves were tightly ligated with 6-0 silk thread. The development of neuropathy was confirmed by daily measurement of the paw withdrawal threshold by using Von Frey filaments (see below). Behavioural experiments were conducted after neuropathy was established.
Paclitaxel-induced neuropathy model
Groups of rats received either vehicle (10% Cremophor EL in saline) or 1.0 mg kg−1 of paclitaxel daily i.p. for four consecutive days for a final cumulative dose of 4 mg kg−1 (Polomano et al., 2001); the injection volume was 1 mL kg−1. Baseline responses to mechanical stimulation of the hind paw (see below) were established on day 0 and continued daily until the development of neuropathy was confirmed.
Assessment of mechanical withdrawal thresholds
Rats were placed in a compartment with a wire mesh bottom, and allowed to acclimatize for a minimum of 30 min before testing. Mechanical sensitivity was assessed by using a series of Von Frey filaments with logarithmic incremental stiffness (0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50 and 15.1 g) (Stoelting Co., Wood Dale, IL, USA), as previously described (Chaplan et al., 1994), and 50% probability withdrawal thresholds were calculated with the up–down method (Dixon, 1965). In brief, beginning with the 2.0-g probe, filaments were applied to the plantar surface of a hind paw for 6–8 s, in an ascending or descending order after a negative or positive withdrawal response, respectively. Six consecutive responses after the first change in response were used to calculate the withdrawal threshold (in grams). When response thresholds fell outside the range of detection, the withdrawal threshold was assigned as 15 g for continuous negative responses and 0.25 g for continuous positive responses. The percentage maximal possible effect (%MPE) was calculated as ((postdrug threshold−baseline threshold)/(cutoff threshold (15 g)−baseline threshold)) × 100. The baseline threshold was determined once. Different groups were tested in the same hour on consecutive days. The only exception is the ‘vehicle+vehicle' group experiment, which was performed on a different day, separate from other groups. The drug groups were not randomized. Only one experimenter performed all behavioural tests and he, therefore, was not unaware of the different treatment groups.
Assessment of paw withdrawal latencies in response to noxious heat
To determine sensitivity to noxious heat, rats were placed in plexiglass enclosures on a transparent glass surface maintained at 30 °C, and allowed to acclimatize for 30 min. A thermal testing apparatus, consisting of a heat-emitting projector lamp and an electronic timer, was used. The device was activated after the lamp was placed directly beneath the plantar surface of the hind paw. The paw withdrawal latency in response to the radiant heat (50 W) was recorded using a digital timer. A cutoff of 30 s was used to prevent tissue damage. After the baseline paw withdrawal response to noxious heat was measured, three groups of naive rats (n=10 per group) received 1, 3 or 10 mg kg−1 of MDA7 i.p. Response latencies were determined for each rat before i.p. drug injection and at 5, 10, 15, 30, 45, 60, 90 and 120 min after i.p. drug injection. Each paw was only stimulated once per time point.
Open-field chamber testing
An automated open-field chamber (ENV-515 Test Environment, Med Associates, St Albans, VT, USA), 43.2 × 43.2 × 30.5 cm (L × W × H), equipped with three pairs of 16 infrared arrays that continually monitored each animal's movement, was used to determine potential CNS effects of MDA7, WIN 55,212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone), and haloperidol in naive rats. Rats were individually tested 15 min after i.p. drug administration. The infrared beams were set 2.5 cm apart horizontally and at a height of 3 cm above the floor, with the rearing array set at 12 cm from the floor. The area in the box was divided into four equal quadrants (zones), with data collected within each quadrant and across quadrants (zone entries). An ambulatory movement was defined as a motion of at least 5 cm and was coded by quadrant. Vertical movements were counted when the rat moves vertically at least 12 cm from the floor. Zone entries were defined as an entry into a zone from another zone. Entry into a zone was counted when the rat was far enough into the new zone to break two sets of new zone photoelectric beams during an ambulatory movement.
Data analysis
Statistical analyses were carried out by using BMDP 2007 (Statistical Solutions, Saugus, MA, USA) and Graph Pad Prism (version 4.03; Graph Pad Software Inc., San Diego, CA, USA). Data were analysed by using one-way ANOVA. If findings on ANOVA were significant, Tukey–Kramer post hoc analysis was used for multiple group comparison. Area under the curve (AUC) was calculated using the trapezoidal rule. The results were presented as mean±s.e.mean and were considered significant at P<0.05. Analyses of the dose–response curves and statistics were obtained by using the pharmacological software programs of Tallarida and Murray (1987), and included calculation of the ED50 values and their 95% confidence intervals (CIs).
Compounds and solutions
AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl)methanone) and AM251 were purchased from Tocris Bioscience. WIN 55,212-2, AM1241, naloxone, paclitaxel and all chemicals used for the synthesis of MDA7 were purchased from Sigma-Aldrich Inc. (St Louis, MO, USA).
WIN 55,212-2, AM1241, AM630, AM251 and haloperidol were administered in vivo in 0.25 mL of 100% DMSO. MDA7 was administered in vivo in a vehicle consisting of N-methylpyrrolidone 25%, propylene glycol 25%, Cremophor ELP 10% and sterile water 40%. Briefly, MDA7 was dissolved in N-methylpyrrolidone. Propylene glycol and Cremophor were added dropwise at 40 °C. After the solution was stirred for 15 min, water was added dropwise. After an additional 15 min of stirring at 40 °C, the solution was cooled to room temperature. By this method, a solution of about 18 mM MDA7 could be prepared, enough for a 150 g rat.
Results
In vitro characterization of MDA7
In the competitive binding assays performed in membranes of CHO-K1 cells selectively expressing the human CB2 receptor, MDA7 potently displaced [3H]CP55,940 from human receptors (Table 1). In the competitive binding assays performed in membranes of CHO-K1 cells selectively expressing human CB1 receptors, MDA7 (up to 3 μM) did not demonstrate detectable radioligand displacement (Table 1). Corresponding Ki values at CHO-K1 cells selectively expressing the rat CB1 and CB2 receptors were about 10-fold higher for the CB1 than for the CB2 receptor (Table 1). In GTPγ[35S] functional assays, MDA7 gave measurable EC50 values (Table 2) at human and rat CB2 receptors, but was without any activity at human or rat CB1 receptors, up to 1 μM. Weak CB1 partial agonist activity was detected at a concentration of more than 1 μM (Figure 2).
Table 1.
Radioligand competitive binding assays
| Ligand |
Mean Ki (nM) |
|||
|---|---|---|---|---|
| Human CB1 | Human CB2 | Rat CB1 | Rat CB2 | |
| MDA7 | >10,000 | 422±123 | 2565±695 | 238±143 |
| CP55,940 | 3.4 | 1.8±1.1 | ND | 1.1±0.02 |
| AM251 | ND | ND | 0.58±0.06 | ND |
Abbreviations: CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; ND, not done.
Table 2.
GTPγ[35S] functional assays
| Ligands |
Agonist EC50 (nM) (mean±s.e. mean) relative to CP55,940 (%) |
|||
|---|---|---|---|---|
|
GTPγ[35S] functional assays |
GTPγ[35S] functional assays |
|||
| Human CB1 | Human CB2 | Rat CB1 | Rat CB2 | |
| MDA7 | NA | 128±32 | NA | 67.4±4.9 |
| CP55,940 | 9±1.3 | 6.5±2.1 | 8.2±1.3 | 2.3±1.8 |
Abbreviations: CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; NA, not active at 1 μM.
Figure 2.
Characterization of CP55,940 (a) and MDA7 (b) in recombinant human cannabinoid types 1and 2 (CB1 and CB2) (hCB1 and hCB2) GTPγ[35S] assay systems. The levels of receptor activation were calculated and were expressed as a percentage relative to the response to 1.0 μM CP55,940.
Effects of MDA7 on thermal nociception in naive rats
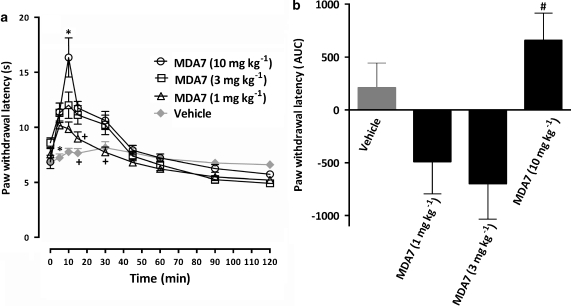
Administration of 1 or 3 mg kg−1 of MDA7 i.p. did not block the nociceptive effect of a thermal stimulus applied to the hind paws of naive rats. Increasing the dose of MDA7 to 10 mg kg−1 i.p. resulted in a short-lasting antinociceptive effect (Figure 3).
Figure 3.
Effects of i.p. administration of MDA7 on the hind paw withdrawal latency in response to noxious heat in naive rats (n=10 per group). (a) The time course of percent maximal possible effect (%MPE) and (b) the area under the curve (AUC) of 1.0, 3.0 and 10 mg kg−1 of MDA7 and the vehicle. *P<0.05 compared with other groups. +P<0.05 compared with 3.0 or 10 mg kg−1 of MDA7 and the vehicle (ANOVA followed by Tukey–Kramer test for multiple group comparison). #P<0.05 compared with 1.0 and 3.0 mg kg−1 of MDA7 (ANOVA followed by Tukey–Kramer test for multiple group comparison). Each point represents the mean±s.e.mean.
Effects of MDA7 on tactile allodynia in the spinal nerve ligation model of neuropathic pain
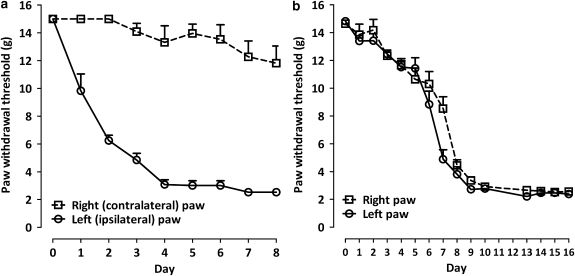
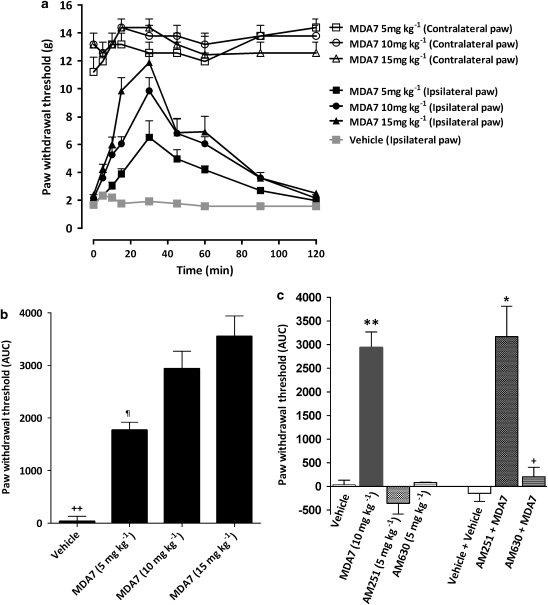
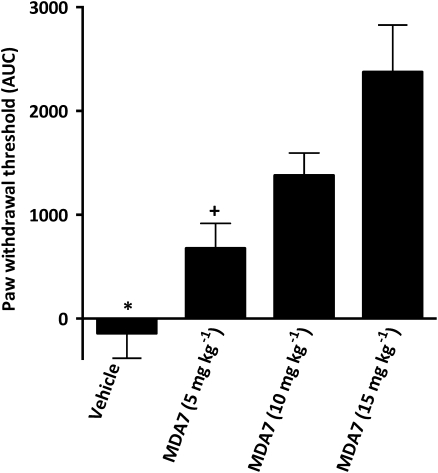
In rats, spinal nerve ligation produced tactile allodynia 1 week after surgery, as demonstrated by a marked reduction in paw withdrawal threshold to mechanical stimulation with Von Frey filaments (Figure 4a). MDA7 (i.p.) attenuated tactile allodynia in a dose-related manner; the ED50 was 7.5 mg kg−1 i.p. (95% CI=5.6–9.9 mg kg−1). The higher doses of MDA7 (10 and 15 mg kg−1) produced a significantly greater antiallodynic effect than that noted with a dose of 5 mg kg−1 (Figure 5).
Figure 4.
Development of tactile allodynia (a) after spinal nerve ligation and (b) after i.p. administration of paclitaxel for 4 days (n=10 per group). Each point represents the mean±s.e.mean.
Figure 5.
Effects of MDA7 and selective antagonists for cannabinoid types 1 and 2 (CB1 and CB2) receptors, administered by i.p., on tactile allodynia in a spinal nerve ligation model of neuropathic pain in rats (n=6 per group). MDA7 induced increases in the withdrawal threshold of the nerve-injured paw in a dose-dependent manner. (a) The time course of 5, 10 and 15 mg kg−1 of MDA7. (b) Area under the curve (AUC). (c) Effects of selective antagonists for CB1 and CB2 receptors on the antiallodynic effects of 10 mg kg−1 of MDA7, shown as AUC (n=6 per group). All drugs were administered by i.p. injection. Administration of 5 mg kg−1 of AM251, a selective CB1 receptor antagonist, or 5 mg kg−1 of AM630, a selective CB2 receptor antagonist, had no effect. Administration of 5 mg kg−1 of AM630 15 min before administration of MDA7 (AM630+MDA7) reversed the antiallodynic effects of MDA7. Administration of 5 mg kg−1 of AM251 15 min before administration of MDA7 (AM251+MDA7) did not affect the antiallodynic effects of MDA7. Data expressed as mean±s.e.mean. ++P<0.001 versus all other groups (ANOVA followed by Tukey–Kramer test for multiple group comparison). ¶P<0.05 versus MDA7 10 mg kg−1 and MDA7 15 mg kg−1 (ANOVA followed by Tukey–Kramer test for multiple group comparison). **P<0.001 versus vehicle, AM251, AM630 (ANOVA followed by Tukey–Kramer test for multiple group comparison). *P<0.001 versus vehicle+vehicle and AM251. +P<0.001 versus MDA7 10 mg kg−1 (ANOVA followed by Tukey–Kramer test for multiple group comparison).
The receptor specificity of MDA7 was investigated in this spinal nerve ligation model using receptor-selective antagonists (Figure 5c). Pretreatment with AM630 (5 mg kg−1 i.p.), a selective CB2 receptor antagonist (Hosohata et al., 1997; Ross et al., 1999), significantly reversed the antiallodynic effects induced by MDA7 (10 mg kg−1 i.p.) (P<0.001). In contrast, pretreatment with AM251 (5 mg kg−1 i.p.), a selective CB1 receptor antagonist (Gatley et al., 1996), did not affect the antiallodynic effects induced by MDA7. The rats treated with CB1 or CB2 receptor antagonists alone at the doses used in these studies exhibited no significant change in paw withdrawal threshold compared with results from the vehicle-treated animals (Figure 5c).
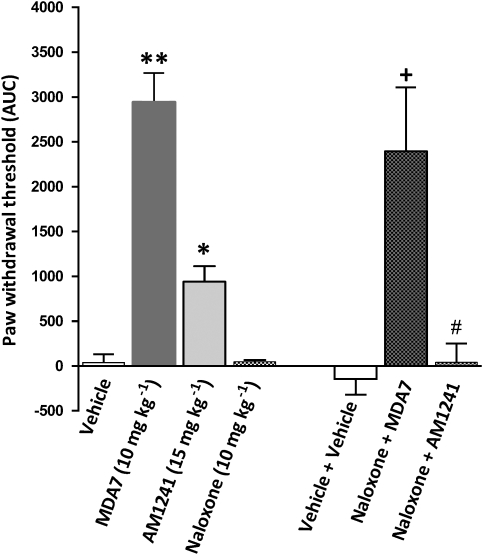
Administration of AM1241 (15 mg kg−1 i.p.), a CB2 ligand (Ibrahim et al., 2003), produced antiallodynic effects that were significantly different (P<0.001) from those of the vehicle (Figure 6). However, the antiallodynic effects of 10 mg kg−1 of MDA7 i.p. were significantly greater (P<0.001) than those that were observed with AM1241. The antinociceptive effects of AM1241 have been shown to involve the μ-opioid receptor system and β-endorphin, and to be blocked by administration of the opioid receptor antagonist naloxone or antiserum to β-endorphin (Ibrahim et al., 2005). In this study, rats subjected to spinal nerve ligation were treated with naloxone (10 mg kg−1 i.p.) 15 min before the administration of MDA7. Naloxone pretreatment had no effect on the antiallodynic activity of MDA7 (Figure 6). However, pretreatment with naloxone significantly reversed the antiallodynic activity of AM1241 (Figure 6, P<0.01), confirming that the antiallodynic effects of MDA7 were not mediated by μ-opioid receptor-dependent activity.
Figure 6.
Effects of the opioid receptor antagonist naloxone on MDA7- and AM1241-induced antiallodynic effects in a spinal nerve ligation model of neuropathic pain in rats (n=6 per group). All drugs were administered by i.p. injection. The antiallodynic effects of MDA7 (10 mg kg−1) were significantly greater than were those of a cannabinoid type 2 (CB2) receptor-selective agonist, AM1241 (15 mg kg−1). Administration of the opioid antagonist naloxone (10 mg kg−1) did not affect the paw withdrawal threshold. Pretreatment with naloxone (10 mg kg−1) followed 15 min later by MDA7 (10 mg kg−1) (naloxone+MDA7) did not affect the antiallodynic effects of MDA7. In contrast, pretreatment with naloxone (10 mg kg−1) followed 15 min later by AM1241 (15 mg kg−1) (naloxone+AM1241) reversed the antiallodynic effects of AM1241. **P<0.001 compared with vehicle, AM1241 and naloxone (ANOVA followed by Tukey–Kramer test for multiple group comparison). *P<0.001 compared with vehicle and naloxone (ANOVA followed by Tukey–Kramer test for multiple group comparison). +P<0.01 compared with vehicle+vehicle and naloxone. #P<0.01 compared with AM1241 (ANOVA followed by Tukey–Kramer test for multiple group comparison).
Effects of MDA7 on tactile allodynia in a paclitaxel-induced neuropathic pain model
Tactile allodynia developed in 100% of rats 10 days after the start of paclitaxel administration, as demonstrated by a reduction in paw withdrawal threshold to mechanical stimulation with Von Frey filaments to 2.9±0.19 and 2.8±0.15 g for the right and left paws, respectively (Figure 4b). In paclitaxel-treated rats, i.p. administration of MDA7 suppressed mechanical allodynia (Figure 7) in a dose-dependent manner, as indicated by an increase in the %MPE withdrawal threshold AUC (Figure 7) and an ED50 of 24 mg kg−1 i.p. The %MPE for reversing mechanical allodynia, observed at 30 min with 15 mg kg−1 of MDA7 i.p., was 42±8.8%.
Figure 7.
Effects of MDA7 administered by i.p. injection on tactile allodynia in a paclitaxel-induced neuropathic pain model in rats (n=8 per group). MDA7 dose-dependently attenuated tactile allodynia in this model, as shown by an increase in the percentage maximal possible effect (%MPE) withdrawal threshold area under the curve (AUC). *P⩽0.05 compared with 10 and 15 mg kg−1 of MDA7 (ANOVA followed by Tukey–Kramer test for multiple group comparison). +P⩽0.05, P<0.05 compared with 15 mg kg−1 of MDA7 (ANOVA followed by Tukey–Kramer test for multiple group comparison).
Open-field chamber testing
In contrast to MDA7 (15 mg kg−1 i.p.), both WIN 55,212-2 (7 mg kg−1 i.p.) (Herzberg et al., 1997) and haloperidol (1 mg kg−1 i.p.) significantly (P<0.05) decreased exploratory behaviour in rats, as shown by a reduction in the total distance travelled (Figure 8a), time spent ambulating (Figure 8b), vertical movements (Figure 8c) and zone entries (Figure 8d).
Figure 8.
Effect of MDA7 on locomotor activity in rats. Exploratory behaviour was tested in an open-field chamber following i.p. administration of vehicle, MDA7, WIN 55,212-2 or haloperidol (n=6 per group). The following parameters were scored for 60 min: distance travelled (a), ambulatory time (b), vertical activity (c) and number of zone entries (d). +P<0.05 versus vehicle and MDA7 (ANOVA followed by Tukey–Kramer test for multiple group comparison). *P<0.05 versus MDA7 (ANOVA followed by Tukey–Kramer test for multiple group comparison).
Discussion
The results of this study indicate that MDA7 is a selective CB2 agonist that suppresses allodynia in rats with spinal nerve ligation or paclitaxel-induced neuropathic pain without affecting their locomotor activity. MDA7 showed good affinity and excellent selectivity for CB2 receptors and low affinity and efficacy at CB1 receptors. The effects of MDA7 were inhibited by the CB2 receptor-selective antagonist AM630 but not by the CB1 receptor-selective antagonist AM251 or by the opioid receptor antagonist naloxone, indicating that the effects of MDA7 are mediated by CB2 receptors.
The CB2 receptor has emerged as a new target for treating neuropathic pain, and CB2 receptor agonists have the advantage of lacking the adverse psychotropic effects normally seen with the CB1 receptor agonists (Beltramo et al., 2006; Cox et al., 2007; Whiteside et al., 2007; Guindon and Hohmann, 2008). The mechanism by which CB2 receptors modulate neuropathic pain is still poorly understood. Peripheral nerve injury induces CB2 protein expression in rat sensory neurons (Wotherspoon et al., 2005). The mRNA for CB2 receptors is expressed in the dorsal root ganglia of neuropathic rats and is upregulated in their spinal cords. Expression of CB2 receptor mRNA was also found in cultured spinal cord microglia (Walczak et al., 2005; Beltramo et al., 2006) and was upregulated in reactive microglia (Ashton and Class, 2007; Romero-Sandoval and Eisenach, 2007).
The efficacy of selective CB2 receptor agonists in attenuating neuropathic pain has been reported with only a few compounds, including A-796260 (Yao et al., 2008), AM1241 (Malan et al., 2001; Ibrahim et al., 2003) and GW405833 (Valenzano et al., 2005). In this study, MDA7 exhibited specificity for human CB2 receptors; indeed CB1 receptor-mediated functional activity for MDA7 was so low that we were not able to calculate the corresponding EC50 for MDA7 at human CB1 receptors. The CB2 agonist GW842166X (Giblin et al., 2007) is scheduled to undergo phase 2 studies in Europe for treatment of thermal pain. It is worth noting that Yao et al. (2008) found that GW842166X exhibits low potency for displacement of [3H]CP55,940 from the human and rat CB2 receptors (2000 and 2580 nM, respectively) yet exhibits full agonist efficacy in functional assays.
In a partial sciatic nerve ligation model of neuropathic pain, Yao et al. (2008) reported that the ED50 for reversal of tactile allodynia for A-796260 was 15 mg kg−1 i.p. (95% CI=5.3–26 mg kg−1), with an efficacy of 66±9% for the highest dose used (35 mg kg−1 i.p.). In the same model, Valenzano et al. (2005) assessed the effects of 0.1, 0.3, 1, 10 and 30 mg kg−1 i.p. of GW405833 on the reversal of mechanical hyperalgesia. The maximum percentage reversal (63.6±9.2%) was achieved 1 h after administration of the 10 mg kg−1 dose. The effect of the 30 mg kg−1 dose of GW405833 was less than that noted with the 10 mg kg−1 dose.
We recently described a series of N-alkyl isatin acylhydrazone derivatives that act as selective CB2 agonists (Diaz et al., 2008). This series was based on a parallel synthesis approach to generate structure-activity relationships. A second novel series based on a racemic mixture of 3,3-disubstituted-2,3-dihydro-1-benzofuran compounds was then designed to increase bioavailability compared with that of the isatin series. Comparing benzofuran with isatin structures, it was assumed that the benzofuran scaffold might mimic the isatin scaffold. In Figure 1a, ring B from the benzofuran series is superimposable on the six-membered ring formed by the hydrazone, and the indolone through an internal hydrogen bond. In this case, both the five-membered rings (ring A) from the isatin and benzofuran structures fit. Structure-activity relationships based on the isatin series have been used to shorten the optimization time for the benzofuran series. The piperidine moiety was used to mimic the cyclohexyl group borne by the hydrazoic moiety in the isatin series (R1 in Figure 1a). Because the isatin compound with a benzyl moiety on the nitrogen of the isatin ring (R2 in Figure 1a) exhibited one of the best ratios in terms of selectivity and activity for CB2 receptors, we retained this substituent in the benzofuran series, resulting in the synthesis of MDA7.
In our spinal nerve ligation model in rats, we noted that MDA7 treatment attenuated tactile allodynia in a dose-related manner, with an ED50 of 7.5 mg kg−1 i.p. (95% CI=5.6–9.9 mg kg−1) and an efficacy of 75.1±5% after the 15 mg kg−1 dose of MDA7 i.p. In the same model, AM1241 had a %MPE of 100% in suppressing allodynia at a dose of 3 mg kg−1 i.p. (Ibrahim et al., 2005). Using the same model in this study, we noted that the %MPE of a larger dose (15 mg kg−1 i.p.) of AM1241 was only 25% at 10 min (data not shown). In fact, the effect of 15 mg kg−1 of AM1241 i.p. was significantly less (P<0.01) than that of 10 mg kg−1 of MDA7 i.p. in suppressing allodynia in the spinal nerve ligation model (Figure 6). In accordance with our results (Figure 6), Yao et al. (2008) noted that the effects of AM1241 were antagonized by naloxone. The antiallodynic effects of MDA7 were not affected by naloxone. It is not clear how AM1241 interacts with the opioid system, as it has no affinity for the μ-opioid receptor (Ibrahim et al., 2005).
In this study, paclitaxel did not affect the baseline paw withdrawal latency in response to thermal stimulation compared with naive rats. Similar observations were previously reported in a vincristine-induced neuropathy model (Rahn et al., 2007). In our study, MDA7 suppressed paclitaxel-induced mechanical allodynia relative to treatment with the vehicle in a dose-dependent manner (Figure 7). The effects of CB2 receptor ligands on acute nociception have not yet been fully explored. Some reports have suggested that some CB2 receptor ligands have antinociceptive effects in naive animals. AM1241 produced dose-dependent antinociception in response to a thermal stimulus in rats and mice (Malan et al., 2001; Ibrahim et al., 2005). These effects were absent in CB2 knockout mice (Ibrahim et al., 2006). The antinociceptive effect was reversed with the CB2 receptor-selective antagonist AM630 (Malan et al., 2001) or with naloxone or antiserum to β-endorphin (Ibrahim et al., 2005). In contrast, administration of the CB2 receptor-selective agonist HU-308 did not affect acute nociception in mice, when thermal withdrawal latency was measured on a 55 °C hot plate (Hanus et al., 1999). Treatment with GW405833 i.p. did not affect hot plate or tail flick latency after doses of up to 30 mg kg−1. However, 100 mg kg−1 of GW405833 resulted in a significant increase in both tail flick and hot plate latencies 1 h after administration (Valenzano et al., 2005). The 100 mg kg−1 dose of GW405833 that resulted in an analgesic effect on tail flick and hot plate test has also been shown to induce typical CB1 receptor-mediated effects (Valenzano et al., 2005)—not supporting a CB2-mediated effect in acute nociception. In this study, low doses of MDA7 (1 and 3 mg kg−1 i.p.) failed to suppress thermal nociception in naive rats. Only higher doses (10 mg kg−1 of MDA7 i.p.) resulted in modest antinociceptive effects (Figure 3).
We used 0.25 mL (0.25 g) of 100% DMSO as a vehicle in this study and observed no analgesic effects. The behavioural effects (motor activity, grip strength, paw pressure, mechanical non-nociceptive thresholds with the von Frey hair test) after repeated i.p. injections of 10 mL kg−1 of various concentrations of DMSO (1.8, 3.6, 7.2%) for 10 days were studied in male Sprague–Dawley rats (Authier et al., 2002). The maximum dose of DMSO used in the latter studies was 0.144 g daily for 10 days (total dose, 1.44 g) for a rat weighing 200 g. Results from behavioural tests were not different between the treated and control groups except for the withdrawal thresholds to von Frey hairs, which demonstrated a slight but significant decrease only after the 10th cumulative dose (Authier et al., 2002). DMSO is known to be a cumulative drug (Hucker et al., 1967). It should be noted that Authier et al. (2002) used von Frey hairs in normal (not neuropathic) rats in their experiments. The reported median motor activity of mice (measured as revolutions per hour of activity wheel) after oral or i.p. administration of 5 g kg−1 of DMSO were 38 and 71.1, respectively, and the corresponding control (saline) values were 271 and 51.1 respectively (Kocsis et al., 1975).
MDA7 did not inhibit ambulation or rearing in the open-field testing. Others studies have shown that other selective CB2 receptor agonists also did not affect locomotor activity (Yao et al., 2008) or cause catalepsy (Hanus et al., 1999).
In summary, we have described the synthesis and pharmacological characterization of a novel selective CB2 agonist, MDA7. We found that MDA7 was effective in treating various models of neuropathic pain in rats by activating CB2 receptors without affecting the locomotor behaviour of the animals. Our study supports the role of selective CB2 ligands in modulating neuropathic pain.
Supplementary Material
Abbreviations
- A-796260
[1-(2-morpholin-4-yl-ethyl)-1H-indol-3-yl]-(2,2,3,3-tetramethylcyclopropyl)-methanone
- AM1241
(2-iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl-methanone
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methy l-1H-pyrazole-3-carboxamide
- AM630
(6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl)methanone)
- CP55,940
5-(1,1-dimethylheptyl)-2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxy-propyl)-cyclohexyl]-phenol
- GW842166X
2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide
- L-768,242 (GW405833)
(2,3-dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-morpholin-4-yl-ethyl)-indol-1-yl]-methanone
- MDA7
1-[(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl]piperidine
- SR141716A
5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1H-pyrazole-3-carboxylic acid piperidin-1-ylamide
- WIN 55,212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de)-1,4-benzoxazin-6-yl]-1-napthalenylmethanone
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA.Guide to receptors and channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–S209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Class M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr neuropharmacol. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Dupuis E, Kwasiborski A, Eschalier A, Coudore F. Behavioural assessment of dimethylsulfoxide neurotoxicity in rats. Toxicol Lett. 2002;132:117–121. doi: 10.1016/s0378-4274(02)00052-8. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, et al. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol. 2007;151:1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI. Br J Pharmacol. 2008;153:367–379. doi: 10.1038/sj.bjp.0707506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S, Bertorelli R, Forlani A, Fredduzzi S, Nicolussi E, Reggiani A. 33 Congresso Nazionale della Societa Italiana di Farmacologia. Societa Italiana di Farmacologia: Cagliari, Sardinia, Italy, p 50; 2007. Role of GW842166X, a CB2 receptor agonist, in several rat models of neuropathic and inflammatory pain. [Google Scholar]
- Cox ML, Haller VL, Welch SP. The antinociceptive effect of [Delta]9-tetrahydrocannabinol in the arthritic rat involves the CB2 cannabinoid receptor. Eur J Pharmacol. 2007;570:50–56. doi: 10.1016/j.ejphar.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Davis MP. What is new in neuropathic pain. Support Care Cancer. 2007;15:363–372. doi: 10.1007/s00520-006-0156-0. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Diaz P, Xu JJ, Astruc-Diaz F, Pan H-M, Brown DL, Naguib M.Design and synthesis of a novel series of N-alkyl isatin acylhydrazone derivatives that act as selective CB2 agonists for the treatment of neuropathic pain J Med Chem 2008. 31 July 2008 [Epub ahead of print]doi [DOI] [PubMed]
- Dixon W. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- Edgar KJ, Falling SN. An efficient and selective method for the preparation of iodophenols. J Org Chem. 1990;55:5287–5291. [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279 Part 1:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin GMP, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, et al. Discovery of 2-[(2,4-Dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007;50:2597–2600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn CA, Fan X, Deno G, Kozlowski J, Hipkin RW. Sch35966 is a potent, selective agonist at the peripheral cannabinoid receptor (CB2) in rodents and primates. Br J Pharmacol. 2007;151:1262–1271. doi: 10.1038/sj.bjp.0707336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts SJ, Hermans E, Lambert DM. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharm Sci. 2004;23:233–243. doi: 10.1016/j.ejps.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Holdcroft A, Maze M, Dore C, Tebbs S, Thompson S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology. 2006;104:1040–1046. doi: 10.1097/00000542-200605000-00021. [DOI] [PubMed] [Google Scholar]
- Hosohata Y, Quock RM, Hosohata K, Makriyannis A, Consroe P, Roeske WR, et al. AM630 antagonism of cannabinoid-stimulated [35S]GTP gamma S binding in the mouse brain. Eur J Pharmacol. 1997;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- Hucker HB, Miller JK, Hochberg A, Brobyn RD, Riordan FH, Calesnick B. Studies on the absorption, excretion and metabolism of dimethylsulfoxide (DMSO) in man. J Pharmacol Exp Ther. 1967;155:309–317. [PubMed] [Google Scholar]
- Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, et al. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. J Med Chem. 1996;39:3875–3877. doi: 10.1021/jm960394y. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, et al. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290:1757–1762. doi: 10.1001/jama.290.13.1757. [DOI] [PubMed] [Google Scholar]
- Khaliq W, Alam S, Puri N. Topical lidocaine for the treatment of postherpetic neuralgia. Cochrane Database Syst Rev. 2007;2:CD004846. doi: 10.1002/14651858.CD004846.pub2. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Ohashi K, Sugie Y, Sugimoto H, Omura H. Pharmacological evaluation of a novel cannabinoid 2 (CB(2)) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci. 2008;106:219–224. doi: 10.1254/jphs.fp0071599. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kocsis JJ, Harkaway S, Snyder R. Biological effects of the metabolites of dimethyl sulfoxide. Ann NY Acad Sci. 1975;243:104–109. doi: 10.1111/j.1749-6632.1975.tb25349.x. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hindpaw incision: model of postoperative pain. Eur J Pharmacol. 2005;527:172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Manera C, Benetti V, Castelli MP, Cavallini T, Lazzarotti S, Pibiri F, et al. Design, synthesis, and biological evaluation of new 1,8-Naphthyridin-4(1H)-on-3-carboxamide and Quinolin-4(1H)-on-3-carboxamide derivatives as CB2 selective agonists. J Med Chem. 2006;49:5947–5957. doi: 10.1021/jm0603466. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Adams M, Whiteaker K, Daza A, Kage K, Cassar S, et al. Species comparison and pharmacological characterization of rat and human CB2 cannabinoid receptors. Eur J Pharmacol. 2004;505:1–9. doi: 10.1016/j.ejphar.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murineddu G, Lazzari P, Ruiu S, Sanna A, Loriga G, Manca I, et al. Tricyclic PYRAZOLES. 4. Synthesis and biological evaluation of analogues of the robust and selective CB2 cannabinoid Ligand 1-(2′,4′-Dichlorophenyl)-6-methyl-N-piperidin-1-yl-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide. J Med Chem. 2006;49:7502–7512. doi: 10.1021/jm060920d. [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ishizaka T, Tatsuzuki M, Yoshinaga M, Iida I, Tomishima Y, et al. N-Alkylidenearylcarboxamides as new potent and selective CB2 cannabinoid receptor agonists with good oral bioavailability. Bioorg Med Chem Lett. 2007a;17:6299–6304. doi: 10.1016/j.bmcl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ishizaka T, Yoshinaga M, Morita A, Tomishima Y, Toda Y, et al. Sulfonamide derivatives as new potent and selective CB2 cannabinoid receptor agonists. Bioorg Med Chem Lett. 2007b;17:5133–5135. doi: 10.1016/j.bmcl.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong J-P, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann NY Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metabol. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Page D, Yang H, Brown W, Walpole C, Fleurent M, Fyfe M, et al. New 1,2,3,4-tetrahydropyrrolo[3,4-b]indole derivatives as selective CB2 receptor agonists. Bioorg Med Chem Lett. 2007;17:6183–6187. doi: 10.1016/j.bmcl.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007;152:765–777. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio PH. Pharmacophores for ligand recognition and activation/inactivation of the cannabinoid receptors. Curr Pharm Des. 2003;9:1607–1633. doi: 10.2174/1381612033454577. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo OMH, Raitio KH, Savinainen JR, Nevalainen T, Lahtela-Kakkonen M, Laitinen JT, et al. Virtual screening of novel CB2 ligands using a comparative model of the human cannabinoid CB2 receptor. J Med Chem. 2005;48:7166–7171. doi: 10.1021/jm050565b. [DOI] [PubMed] [Google Scholar]
- Szlosek-Pinaud M, Diaz P, Martinez J, Lamaty F. Efficient synthetic approach to heterocycles possessing the 3,3-disubstituted-2,3-dihydrobenzofuran skeleton via diverse palladium-catalyzed tandem reactions. Tetrahedron. 2007;63:3340–3349. [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs 1987Springer-Verlag: New York; 2nd edn [Google Scholar]
- Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48. doi: 10.1016/j.clinthera.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18:355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132:1093–1102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154–163. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Lee GP, Valenzano KJ. The role of the cannabinoid CB2 receptor in pain transmission and therapeutic potential of small molecule CB2 receptor agonists. Curr Med Chem. 2007;14:917–936. doi: 10.2174/092986707780363023. [DOI] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, et al. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.