Summary
Targeting exposed anionic phospholipids on a spectrum of virus-infected cells can protect against lethal virus infections in vivo.
Keywords: phosphatidylserine, antiviral, therapeutic
There is a pressing need for anti-viral agents that are effective against multiple classes of viruses. Broad specificity might be achieved by targeting phospholipids that are widely expressed on infected host cells or viral envelopes. We reasoned that events occurring during virus replication (e.g. cell activation or pre-apoptotic changes) would trigger the exposure of normally-intracellular anionic phospholipids on the outer surface of virus-infected cells. A chimeric antibody, bavituximab, was used to identify and target the exposed anionic phospholipids. Infection of cells with Pichinde virus (a model for Lassa fever virus, a potential bioterrorism agent) led to the exposure of anionic phospholipids. Bavituximab treatment cured overt disease in guinea pigs lethally infected with Pichinde virus. Direct clearance of infectious virus from the blood and antibody-dependent cellular cytotoxicity of virus-infected cells appeared to be the major anti-viral mechanisms. Combination therapy with bavituximab and ribavirin was more effective than either drug alone. Bavituximab also bound to cells infected with multiple other viruses and rescued mice with lethal murine cytomegalovirus infections. Targeting exposed anionic phospholipids with bavituximab appears to be safe and effective. Our study demonstrates that anionic phospholipids on infected host cells and virions may provide a novel target for the generation of anti-viral agents.
Phosphatidylserine (PS), the most abundant anionic phospholipid of the plasma membrane, is segregated to the inner leaflet of the plasma membrane of resting mammalian cells1,2. This internal positioning of PS is maintained by ATP-dependent aminophospholipid translocases that catalyze aminophospholipid transport from the external to the internal leaflet of the plasma membrane3. Loss of PS asymmetry occurs during apoptosis, cell injury, cell activation, and malignant transformation4, and results from inhibition of the translocases or activation of PS exporters, or lipid scrambling enzymes, such as scramblases5,6.
Two lines of reasoning lead us to hypothesize that exposure of PS and possibly other anionic phospholipids would commonly occur on the surface of virus infected cells. First, viruses, including herpesviruses, Hepatitis C, HIV-1, Pichinde, Machupo and vaccinia, activate host cells to replicate efficiently7–10. Virus-induced cell activation may lead to rises in intracellular Ca2+ that, in turn, cause PS externalization by activating PS exporters and inhibiting PS import by translocases4. This prediction should apply to both enveloped and non-enveloped viruses. Second, PS translocation to the external host cell surface could be an early event associated with virus-induced apoptosis, as occurs with influenza A11, HIV-112, HSV-113 and vaccinia viruses14
To detect and target exposed anionic phospholipids, we used a mouse monoclonal IgG3 antibody, 3G4, which binds with high affinity to complexes of the PS-binding plasma protein β2-glycoprotein I (β2GP1) and anionic phospholipids15. The antibody binds to PS-expressing membranes by crosslinking two molecules of β2GP1 bound to PS on the membrane16. The 3G4-β2GP1-PS complex is only stably formed on PS surfaces. A chimeric version of 3G4, bavituximab, has been generated having mouse 3G4 VH and Vκdomains joined to human IgG1 κ constant domains.
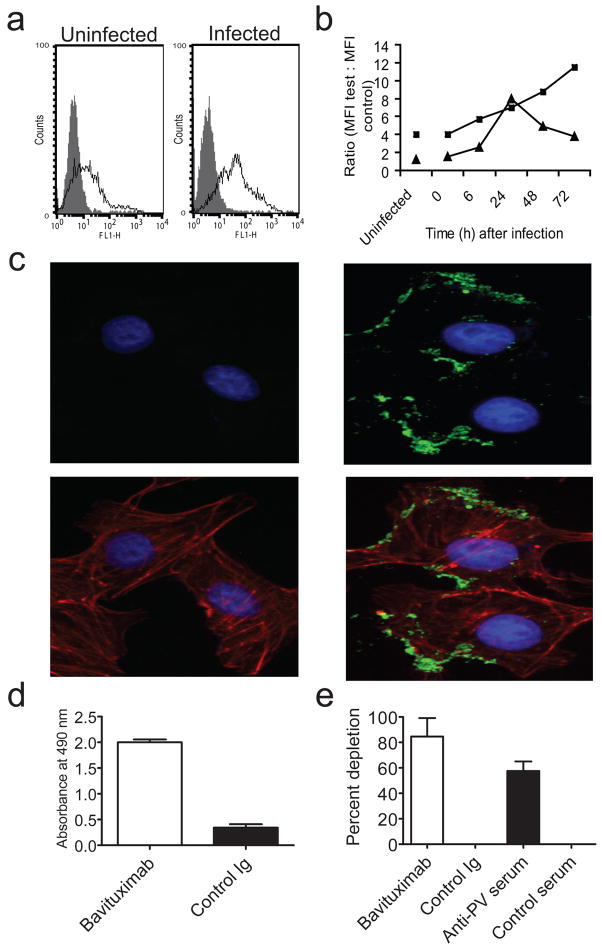
Pichinde is an arenavirus that causes lethal hemorrhagic fever in guinea pigs, closely resembling Lassa fever17 in man. We first determined whether infection of P388D1 cells by Pichinde virus induces PS exposure on the cell surface by flow cytometry. Anionic phospholipids began to appear on the cell surface 6 h after infection, at about the same time as viral antigens, and became progressively stronger over 72 h (Figs. 1a). Immunofluorescence microscopy showed Pichinde infection induces a faint, diffuse plasma membrane staining together with intensely stained discrete regions resembling membrane blebs (Fig. 1c,b and Supplementary Fig. 1 online). Next, we determined whether Pichinde virions carry external PS on their envelopes. Virus suspensions were incubated with ELISA plates coated with bavituximab or control Ig. Selective binding to bavituximab-coated pates was observed (Fig. 1d). Bead depletion studies established that bavituximab binds to infectious virions. Bavituximab-coated magnetic beads specifically removed infectious Pichinde virus, confirming that infectious virions carry external PS (Fig. 1e).
Figure 1. Bavituximab binds to Pichinde virus-infected cells and to infectious virions.
(a) Flow cytometric analysis of P388D1 cells after infection at an moi of 5. Cells were stained at 48 h with bavituximab (open histograms) or control Ig (filled histograms). Live cells were gated based on their exclusion of 7-AAD. (b) Flow cytometric analysis of expression of PS and Pichinde virus antigens over time. P388D1 cells were infected with Pichinde virus at an moi of 5 and stained at the indicated time points after infection. PS expression is detected with bavituximab (closed squares) and Pichinde virus antigen expression is detected with guinea pig Pichinde-specific antiserum (closed triangles). Points, ratio of mean fluorescence intensity (MFI) for cells stained with test antibodies to MFI of cells stained with appropriate negative control antibodies. The error on the points is ± 15%. (c) Immunofluorescence staining of uninfected Vero cells (left panels) and Pichinde virus-infected cells (right panels) with bavituximab 48 h after infection. Upper panels show cells stained with bavituximab (green). Lower panels show images merged with cytoskeleton (red). Nuclei are in blue (all panels). Control Ig did not stain and is not shown. Bar, 50 μm. (d) ELISA detection of virus binding to immobilized bavituximab or control Ig. Columns, average absorbance (n = 3); bars, s.e.m. P<0.0001 (unpaired t test) (e) Depletion of infectious Pichinde by bavituximab-coated magnetic beads. Beads coated with antibodies to Pichinde virus (PV) were used as a positive control. Columns, % depletion (n = 3); bars, s.e.m. P = 0.002 (unpaired t-test).
The therapeutic activity of bavituximab was determined in guinea pigs with advanced Pichinde virus infections. Outbred guinea pigs were treated with bavituximab or isotype-matched control antibody 6–7 d after infection, when the guinea pigs displayed loss of body weight, ruffled fur, and elevated body temperature. All guinea pigs in the control groups had to be euthanatized by day 14–18. In contrast, 50% of the bavituximab-treated guinea pigs recovered. They gained weight and within a week lacked physical signs of disease, and were healthy on day 135 when the experiments were terminated (Fig. 2a). This is the first report of a therapeutic effective against advanced Pichinde virus infections. 14 d after infection, virus loads decreased in blood, spleen, lung, liver, kidney and heart from bavituximab-treated guinea pigs compared to control Ig-treated animals (Fig. 2b). Decreases in virus load were not seen before day 14 (Supplementary Fig. 2 online). By day 135, the bavituximab-treated survivors had completely cleared virus from their tissues (not shown). Combining bavituximab and ribavirin (the drug of choice for treating Lassa fever) had additive activity, as expected for drugs with non-overlapping mechanisms of action. With the combined treatment, 63% of guinea pigs survived, compared to 39% and 35% of animals treated with ribavirin or bavituximab alone, respectively (Fig. 2c).
Figure 2. Therapeutic effect against Pichinde virus in lethally infected guinea pigs displaying overt signs of disease.
(a) Kaplan-Meier survival curves of guinea pigs afterlethal infection with Pichinde virus and treatment with bavituximab or control Ig. Bavituximab or control Ig (6 mg kg−1) was administered i.p. to groups of guinea pigs (n = 8), beginning after they had developed disease signs (around day 7) and three times a week thereafter. The results are representative of those in five separate experiments. Survival in the bavituximab group was significantly superior to the control Ig group (P = 0.0036, Log-rank Mantel Cox test). (b) Virus load in tissues of treated guinea pigs 14 d after infection. Columns, average PFU per gram of tissue (n = 3); bars, s.e.m. The results are representative of two separate experiments. *P = 0.0164, **P = 0.0361, ***P = 0.0436, ****P = 0.0139, *****P = 0.0992, ******P = 0.038. (c) Additive effects of bavituximab and ribavirin treatment. Kaplan-Meier survival curves are shown for Pichinde virus-infected guinea pigs treated with bavituximab and ribavirin (n = 19), bavituximab (n = 20), ribavirin (n = 18) or control Ig (n = 20). Bavituximab or control Ig (6 mg kg−1) was administered i.p. three times per week and ribavirin (8 mg kg−1) was administered i.p. daily, beginning after the guinea pigs developed disease signs. The combination was significantly more effective than bavituximab alone (P = 0.011). All treatments were significantly different from control Ig (P<0.0001). The results are representative of two separate experiments.
The protective effect of bavituximab does not appear to be due to direct neutralization. Bavituximab only inhibits Pichinde-virus replication by 60% in virus-yield assays in vitro, even in physiological concentrations of β2GP1 (Supplementary Fig. 3a online). Thus, PS on Pichinde virus may not be required for virus entry, in contrast to vaccinia14 where viral PS is required for infectivity14. Also, protection is not due to induction of neutralizing antibodies to Pichinde virus (Fig. 3a and Supplementary Fig. 4a online) or Pichinde virus specific T-cells (Fig. 3b and Supplementary Fig. 4b) as neither was detectable 6–7 d after onset of treatment (14 d after infection) when the treated animals begin to recover. Two mechanisms appear to explain the protective effect: 1) bavituximab causes opsonization and clearance of infectious virus from the bloodstream, leaving less virus to infect other tissues. Bavituximab treatment of viremic guinea pigs reduced infectious virus in the bloodstream by 95% within 24 h (Fig. 3c); 2) bavituximab induces antibody-dependent cellular cytotoxicity (ADCC) of virus-infected cells. Bavituximab mediates lysis of virus-infected primary guinea pig fibroblasts by primary guinea pig macrophages in vitro (Fig. 3d). Since PS exposure is an early event during virus infection, ADCC may limit virus spread.
Figure 3. Mechanism of anti-viral effects of bavituximab.
(a) Lack of Pichinde virus-specific humoral response in bavituximab-treated guinea pigs. Plasma from Pichinde virus-infected animals (n = 3) was collected 7 d after onset of treatment (14 d after infection). Antibodies (IgG and IgM) to Pichinde virus were quantified by ELISA. The titer of serum from bavituximab-treated guinea pigs was not significantly different from that of control Ig-treated guinea pigs. Points, average absorbance (n = 3); bars, s.e.m. (b) Lack of Pichinde virus antigen-specific proliferative response in splenocytes from bavituximab-treated guinea pigs. Spleens from bavituximab- or control-treated Pichinde virus-infected animals (n = 3) were removed 7 d after onset of treatment (14 d after infection). Splenocytes were stimulated with Pichinde virus antigen or mock antigen and their ability to incorporate [3H]-thymidine was determined. Bavituximab treatment did not significantly increase the stimulation index (SI). Columns, average SI (n = 3); bars, s.e.m. (c) Clearance of Pichinde virus from blood of guinea pigs treated with bavituximab. Blood samples from groups of 4 guinea pigs were harvested 1 d after treatment with bavituximab or control Ig. P = 0.0145 (unpaired t-test). Columns, average PFU per ml (n = 3); bars, s.e.m. (d) Bavituximab mediates ADCC of Pichinde virus-infected guinea pig kidney fibroblasts 48 h after infection. Specific lysis was determined by quantifying 51Cr release. Bavituximab induced specific lysis of virus infected cells, P < 0.001 (unpaired t-test). Columns, average percentages (n = 3); bars, s.e.m.
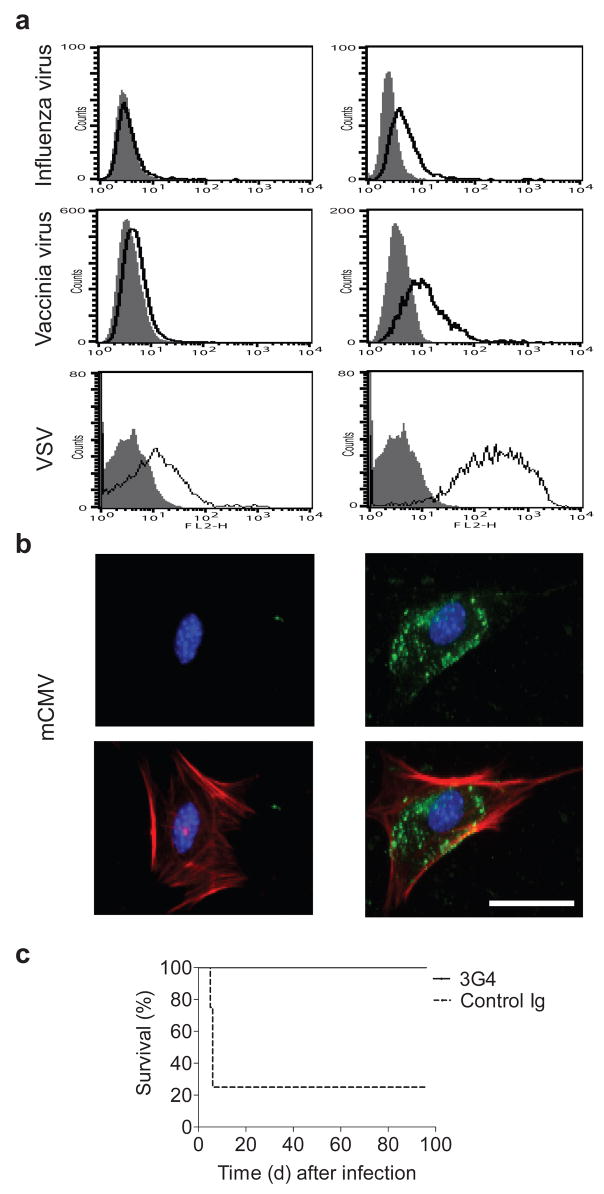
To explore whether PS exposure is a common feature of virus-infected cells, we tested bavituximab binding to cells infected with influenza A, vaccinia, vesicular stomatitis virus (VSV) and murine CMV. All four viruses induced PS exposure as detected by flow cytometry (Fig. 4a) or fluorescence microscopy (Fig. 4b and Supplementary Fig. 5 online). This accords with previous studies using annexin V, showing PS externalization on the surface of cells infected with influenza A18, HIV-112, 19, HSV-113 and vaccinia14. Fluorescence microscopy showed that infection induced diffuse staining of the plasma membrane and the formation of membrane blebs, like those on Pichinde virus infected cells (Fig. 4b and Supplementary Fig. 5).
Figure 4. Broad spectrum recognition of virus infected cells and protection against cytomegalovirus infection in mice.
(a) Flow cytometric analysis of virus-infected cells (right panels) and control uninfected cells (left panels). Cells were infected at an moi of 5. Cells were harvested (at 24 h for influenza A virus and VSV and at 48 h for Vaccinia virus) and stained with bavituximab (open histograms) or control Ig (filled histograms). Live intact cells were gated based on their exclusion of 7-AAD. (b) Immunofluorescence staining of mCMV-infected cells (right panels) and uninfected cells (left panels) with bavituximab. M2-10B4 cells on chamber slides were infected with mCMV at an moi of 5 and stained with bavituximab 48 h later. Upper panels show cells stained with bavituximab (green). Bavituximab is binding to externalized PS on infected cells. Lower panels show images merged with cytoskeleton (red). Nuclei are in blue (all panels). Control Ig did not stain and is not shown. Bar, 50 μm. (c) Kaplan-Meier survival curves of BALB/c mice after infection with an LD80 dose of mCMV and treatment with murine 3G4 (n = 10) or control Ig (n = 8). Treatment with 3G4 (4 mg kg−1) or control Ig was initiated 18 h after infection and administered three times a week thereafter. Survival in the 3G4 group was significantly superior to the control Ig group (P < 0.0001). The results are representative of two separate experiments.
PS also appears to be commonly exposed on enveloped virions. As with Pichinde virus, we found that bavituximab-coated beads specifically bound to and removed infectious VSV virions from suspensions (Supplementary Fig. 6 online). Others have reported that annexin V binds to the external surface of HIV-119 and vaccinia virus14. Many non-icosohedral viruses that bud out from the plasma membrane are assembled in, and bud out of, membrane microdomains, commonly called ‘rafts’ 20. HIV-121, influenza22, VSV23, Moloney murine leukemia virus23, Ebola24, Marburg24, and respiratory syncytial25 viruses all appear to egress from rafts. PS is highly enriched in rafts26 and may stabilize raft formation27. Co-capping and colocalization studies have demonstrated that PS is present on the outer surface of rafts in activated B-cells28 and activated neutrophils29. Possibly, in cells activated to expose PS by virus infection, PS likewise becomes incorporated into raft outer surfaces, from where it is assimilated into the outer viral envelope during budding and egress. Also, the herpesviruses, CMV, HSV-1 and HSV-2, which acquire their envelopes from intracellular organelles, have externalized PS30,31. Herpesviruses obtain their final envelope when they bud into vesicles derived from the trans-Golgi network32. Perhaps Golgi-associated structures into which the virions bud have not yet developed full lipid asymmetry, or they lose PS asymmetry along with the plasma membrane during virus infection.
Our observation that PS is externalized on CMV-infected cells prompted us to test 3G4 against lethal mCMV infections. Bavituximab does not directly neutralize mCMV in vitro (Supplementary Fig. 3b). BALB/c mice were infected with an LD80 dose of mCMV and treated with 3G4 18 h later. Most control mice had to be euthanatized by day five, and only 25% were alive at day 96 (Fig. 4c). In contrast, 3G4 treated mice initially lost weight but then recovered and all were alive at day 96 when the experiment was terminated. These results suggest that the protective effect of bavituximab is not unique to Pichinde virus and/or guinea pigs.
Bavituximab therapy appears to be well-tolerated. Treated animals retained normal body weight, appetite, appearance and physical activity. No evidence of toxicity was visible histologically. Coagulation parameters remained within the normal range (Supplementary Fig. 7 online). 3G4 recognizes domain II of β2GP1, which is not known to be associated with anti-phospholipid syndromes33. In Phase I clinical studies, bavituximab treatment of patients with chronic hepatitis C infections appeared safe and well tolerated. Reductions in serum HCV RNA levels were observed34,35.
PS on virions and virally infected cells may enable viruses to evade immune recognition and dampen inflammatory responses to infection. Viruses may have subverted physiological mechanisms by which apoptotic cells avoid inducing inflammation and autoimmunity. PS suppresses activation and maturation of dendritic cells36, inhibits inflammatory responses of macrophages37,38 and adaptive immune responses in vivo 39,40. Bavituximab treatment may mask PS on virus-infected cells and/or viruses, leading to the development of effective anti-viral immune responses.
In conclusion, targeting PS on cells infected with multiple different viruses and on virions themselves shows promise as an anti-viral strategy. Because anionic phospholipids on virus-infected cells are host-derived and independent of the viral genome, the acquisition of drug resistance should be less problematic than with agents that target virus-encoded components.
Materials and Methods
Flow cytometry
We infected non-adherent P388D1 cells at a multiplicity of infection (moi) of 5 (adherent cells could not be used because cell detachment disturbs PS distribution in the membrane). We washed the cells with FACS buffer (5% FBS, 0.01% sodium azide in PBS) and blocked Fc receptors with mouse serum. We stained the cells with bavituximab and human β2GP1 at 4 °C. We used rabbit Pichinde-specific antiserum to detect Pichinde antigen. Cells were washed and incubated with FITC or PE conjugated antibodies. 7-AAD (BD Biosciences) was added before analyzing cells on a FACScan (Becton Dickinson). Results were analyzed using CellQuest (Becton Dickinson).
Immunofluorescence staining
Cells growing on chamber slides (BD Biosciences) were infected with virus (moi of 5) and stained with bavituximab or control Ig at 37 °C in the presence of β2GP1. We fixed the cells with 4% paraformaldehyde and incubated them with FITC-labeled antibodies to human IgG. We permeabilized the cells with 0.1% Triton-X100 and stained the cytoskeleton with Texas red-labeled phalloidin (Invitrogen) and nuclei with Hoechst 33342 (Invitrogen). Images were captured using a Coolsnap digital camera and analyzed using MetaVue software (MDS Analytical Technologies).
Virus binding studies
For the virus ELISA, we coated Immulon plates with Pichinde virus, washed and blocked. Bavituximab or control antibody was added in the presence of β2GP1. Binding was detected using HRP-labeled antibodies to human IgG and substrate O-phenylenediamine dihydrochloride. The absorbance was measured at 490 nm.
To detect binding of bavituximab to infectious Pichinde virus, we coated Magprep anti-human IgG beads (Novagen) with bavituximab or control Ig. Magprep-SA beads were coated with biotinylated antibodies to guinea pig IgG and guinea pig antibodies to Pichinde virus. We added the beads to virus in the presence of β2GP1 and incubated at 37 °C on a rotator. The beads, together with bound virus, were removed using a magnet. Virus remaining in the supernatant was quantified by plaque assay. Percentage removal of virus was calculated.
Animal studies
We infected male Hartley guinea pigs (Charles River Labs) i.p. with a lethal dose of Pichinde virus. Treatment was initiated after the onset of fever (usually 7 d after infection). For single agent therapy, we treated the animals i.p. with 6 mg kg−1 of bavituximab or control antibody three times a week. For combination therapy, we treated the animals i.p. with 6 mg kg−1 of bavituximab or control antibody three times a week and with 8 mg kg−1 ribavirin daily. Animals were euthanatized when their body weights decreased by greater than 20% or when scored as ‘severe’ based on appearance, clinical signs or unprovoked behavior in accordance with UT Southwestern’s Institutional Animal Care and Use Committee guidelines.
We infected female BALB/c mice (National Cancer Institute) i.p. with an LD80 dose of mCMV. Mice were treated i.p. with 4 mg kg−1 of 3G4 or control Ig beginning 18h after infection and three times a week thereafter. The mice euthanatized when scored as “severe” in accordance with UT Southwestern guidelines.
Tissue virus loads
Animals were sacrificed 14 d after infection. Blood and major organs were frozen. Pichinde virus infects all organs other than the brain and salivary gland. We quantified virus by plaque assay17. Prior experiments had shown that bavituximab at the concentrations present in the blood had no effect on plaque formation.
Antibody-mediated cellular cytotoxicity
We used primary guinea pig kidney fibroblasts as targets and peritoneal exudates from thioglycolate-treated guinea pigs as effectors. For the assay, fibroblasts were infected with Pichinde virus (moi of 5). Bavituximab or control antibody was added to the cultures 24 h after infection in the presence of β2GP1. Effector cells were added 48 h after infection. Cytotoxicity was determined after 18 h using the CytoTox 96 non-radioactive assay (Promega).
Proliferative responses to Pichinde virus antigens
We prepared Pichinde virus antigen or mock antigen by multiple freezing and thawing of extracellular Pichinde virus or mock infected medium. We distributed splenocytes into 96-well round bottomed plates at 5 × 104 cells per well in RPMI 1640 medium containing 10% FBS. We added Pichinde virus antigen (10 μg ml−1) or mock antigen to the cells. On day 3, the cells were pulsed with 1 μCi per well of [3H]-thymidine (Amersham Biosciences). Stimulation index was calculated.
Pichinde-virus specific humoral responses
We tested guinea pig sera for virus-specific antibodies by ELISA. We coated Immulon 4 plates (Dynatech) with Pichinde virus antigen or mock antigen, washed and incubated with guinea pig serum. Binding was detected using peroxidase-conjugated goat antibodies to guinea pig IgG + IgM (Jackson Laboratories Inc.) followed by substrate O-phenylenediamine dihydrochloride. The absorbance was measured at 490 nm.
Statistical analysis
We calculated the significance between two means using Student’s unpaired t-test. Survival data were analyzed using the Mantel-Cox log rank test. P ≤ 0.05 was considered significant.
Supplementary Material
Note: Supplementary information is available on the Nature Medicine website.
Acknowledgments
This work was supported by a US National Institutes of Health grant (5 U01 AI1056412) and a sponsored research agreement with Peregrine Pharmaceuticals, Inc. (Tustin, CA). We thank G. Barbero, S. Mims, H. Arizpe, L. Ingram, S. Syed, L. Watkins, S. Li and J. Iglehart for technical assistance. We also thank W. Bresnahan, S. Ran, O. Ramilo, H. Jafri, S. Fussey, M. Roth, and J. Albanesi for discussions and comments on the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
M.M.S designed and supervised experiments, interpreted results and wrote the manuscript. S.W.K provided reagents and contributed to experimental design and interpretation of results. P.E.T conceived the idea of PS-directed antiviral therapy, oversaw experiments and wrote the manuscript.
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/Reprints and permissions information is available at http://npg.nature.com/Reprintsandpermissions
References
- 1.Williamson P, Schlegel RA. Back and forth: the regulation and function of transbilayer phospholipid movement in eukaryotic cells. Mol Membr Biol. 1994;11:199–216. doi: 10.3109/09687689409160430. [DOI] [PubMed] [Google Scholar]
- 2.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 3.Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984;81:3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 5.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 7.Simmons A, Aluvihare V, McMichael A. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity. 2001;14:763–777. doi: 10.1016/s1074-7613(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 8.Rawls WE, Banerjee SN, McMillan CA, Buchmeier MJ. Inhibition of Pichinde virus replication by actinomycin D. J Gen Virol. 1976;33:421–434. doi: 10.1099/0022-1317-33-3-421. [DOI] [PubMed] [Google Scholar]
- 9.Lukashevich IS, Lemeshko NN, Shkolina TV. Effect of actinomycin D on the reproduction of the Machupo virus. Vopr Virusol. 1984;29:569–572. [PubMed] [Google Scholar]
- 10.Choe W, Volsky DJ, Potash MJ. Activation of NF-kappaB by R5 and X4 human immunodeficiency virus type 1 induces macrophage inflammatory protein 1alpha and tumor necrosis factor alpha in macrophages. J Virol. 2002;76:5274–5277. doi: 10.1128/JVI.76.10.5274-5277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takizawa T, et al. J Gen Virol. 1993;74(Pt 11):2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 12.Banki K, Hutter E, Gonchoroff NJ, Perl A. Molecular ordering in HIV-induced apoptosis. Oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J Biol Chem. 1998;273:11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- 13.Gautier I, Coppey J, Durieux C. Early apoptosis-related changes triggered by HSV-1 in individual neuronlike cells. Exp Cell Res. 2003;289:174–183. doi: 10.1016/s0014-4827(03)00258-1. [DOI] [PubMed] [Google Scholar]
- 14.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 15.Ran S, et al. Clin Cancer Res. 2005;11:1551–1562. doi: 10.1158/1078-0432.CCR-04-1645. [DOI] [PubMed] [Google Scholar]
- 16.Luster TA, et al. Plasma protein beta-2-glycoprotein 1 mediates interaction between the anti-tumor monoclonal antibody 3G4 and anionic phospholipids on endothelial cells. J Biol Chem. 2006;281:29863–29871. doi: 10.1074/jbc.M605252200. [DOI] [PubMed] [Google Scholar]
- 17.Jahrling PB, Hesse RA, Rhoderick JB, Elwell MA, Moe JB. Pathogenesis of a pichinde virus strain adapted to produce lethal infections in guinea pigs. Infect Immun. 1981;32:872–880. doi: 10.1128/iai.32.2.872-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RT, Lichtenberg B, Rick O. Involvement of annexin V in the entry of influenza viruses and role of phospholipids in infection. FEBS Lett. 1996;392:59–62. doi: 10.1016/0014-5793(96)00783-1. [DOI] [PubMed] [Google Scholar]
- 19.Callahan MK, et al. J Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- 20.Briggs JAG, Wilk T, Fuller SD. Do lipid rafts mediate virus assembly and pseudotyping? J Gen Virol. 2003;84:757–768. doi: 10.1099/vir.0.18779-0. [DOI] [PubMed] [Google Scholar]
- 21.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98:13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 23.Pickl WF, Pimentel-Muinos FX, Seed B. Lipid rafts and pseudotyping. J Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavari S, et al. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med. 2002;195:593–602. doi: 10.1084/jem.20011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown G, Rixon HW, Sugrue RJ. Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J Gen Virol. 2002;83:1841–1850. doi: 10.1099/0022-1317-83-8-1841. [DOI] [PubMed] [Google Scholar]
- 26.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 27.Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93:4307–4318. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164:1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 29.Frasch SC, et al. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland MR, Raynor CM, Leenknegt H, Wright JF, Pryzdial EL. Coagulation initiated on herpesviruses. Proc Natl Acad Sci U S A. 1997;94:13510–13514. doi: 10.1073/pnas.94.25.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryzdial EL, Wright JF. Prothrombinase assembly on an enveloped virus: evidence that the cytomegalovirus surface contains procoagulant phospholipid. Blood. 1994;84:3749–3757. [PubMed] [Google Scholar]
- 32.Sanchez V, Spector DH. Virology. CMV makes a timely exit. Science. 2002;297:778–779. doi: 10.1126/science.1075102. [DOI] [PubMed] [Google Scholar]
- 33.de Laat B, Derksen RH, van Lummel M, Pennings MT, de Groot PG. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood. 2006;107:1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 34.Godofsky EW, Shan J. Phase 1 single dose study of bavituximab, a chimeric anti-phosphatidylserine monoclonal antibody, in subjects with chronic hepatitis C. Hepatology. 2006;44:236A. [Google Scholar]
- 35.Lawitz E, Godofsky E, Shan J. Multiple dose safety and pharmacokinetic study of bavituximab, a chimeric anti-phosphatidylserine monoclonal antibody, in subjects with chronic hepatitis C virus (HCV) infection. Hepatology. 2007;46:257A. [Google Scholar]
- 36.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 37.Fadok VA, et al. PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman PR, et al. Interaction between Phosphatidylserine and the Phosphatidylserine Receptor Inhibits Immune Responses In Vivo. Journal of Immunology. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson TA, et al. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 40.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Medicine website.