Abstract
The room temperature synthesis of β-Ga2O3 nanocrystal was examined by coupling two biomimetic crystallization techniques, the enzymatic peptide nano-assembly templating and the aggregation-driven crystallization. The catalytic template of peptide assembly nucleated and mineralized primary β-Ga2O3 crystals, and then fused them to grow single-crystalline and monodisperse nanoparticles in the cavity of the peptide assembly at room temperature. In this work, the peptide assembly was exploited as a nano-reactor with an enzymatic functionality catalyzing the hydrolysis of gallium precursors. In addition, the characteristic ring-structure of peptide assembly is expected to provide an efficient dehydration pathway and the crystallization control over the surface tension, which are advantageous for the β-Ga2O3 crystal growth. This multifunctional peptide assembly could be applied for syntheses of a variety of nanomaterials that are kinetically difficult to grow at room temperature.
Keywords: Self-assembly, Bionanotechnology, Biomineralization, Peptide, Nanoreactors
INTRODUCTION
Recently, nanostructured semiconductor oxides have been attracting a great deal of attention for their potential uses in optoelectronic and sensor applications.1–3 In particular, monoclinic gallium oxide, β-Ga2O3, has been studied extensively because of the wide band gap that provides light emission in a broad range. In general, oxide semiconductor materials are synthesized at high temperature.4 However, if these syntheses can be conducted in milder conditions such as room temperature, it reduces the production cost, the facility size (such as cooling systems), and the manpower, which will give a significant impact on manufacturing gallium-based nanodevices. The low-temperature processing could also lead to reduce nanoparticle aggregations and defects induced from the local thermal stresses. Here, the monodisperse gallium oxide semiconductor crystals, which are known to be kinetically unfavored to grow in ambient conditions, were synthesized at room temperature by coupling two biomimetic crystallization techniques of the enzymatic peptide nano-assembly templating and the aggregation-driven crystallization. The term, aggregation-driven crystallization, is defined as a process in which a single crystal is grown by the aggregation in an aligned manner and the fusion of primary nanoparticles.5 The peptide assembly used in this study captured the nucleated primary particles, mineralized them to β-Ga2O3 crystals using its catalytic functions, and then fused them to grow single-crystalline and monodisperse nanoparticles in the cavity of the peptide assembly at room temperature.
Biological systems possess the function to synthesize exotic materials at room temperature via their enzymatic activities. Recently various room-temperature material syntheses were examined using biomimicked systems engaging biomineralization.6–14 Those biomolecular catalysts, dictated by the genetic code and protein assembly, can facilitate mineralizations via specific interactions between chemical moieties in unique conformations and solutes. This specific interaction that stabilizes and controls the kinetics of the intermediate phases during the synthesis leads to yield in unique crystal structures.15 Although enzymes in nature can mineralize metals and semiconductors whose growths are hardly achieved at room temperature, those enzymes are not exactly designed to grow them in uniform size and shape. For example, silicatein moieties, the silica skeletal elements of a marine sponge, were applied to catalyze the hydrolysis and the polycondensation of γ-Ga2O3.15 In this biomimetic approach, the hydrolytic enzyme motif from the silicateins, consisting of nucleophilic hydroxyl of serine and amine of histidine, catalyzed the hydrolysis of the gallium precursors and resulted in both GaOOH and γ-Ga2O3 nanocrystal formation with a wide size range.6 Although the synthesis of γ-Ga2O3 at room temperature was notable, the size and shape controls were hardly achieved, which could limit its industrial applications. Therefore, it is desirable to develop improved biomimetic templating systems with catalytic activities that can control the shape and the structure of semiconductor nanoparticles simultaneously.
Previously, we have discovered that the peptide assemblies templated and grew metal nanoparticles inside.16 Recently, peptide assemblies also hydrolyzed BaTi(O2CC7H15)[OCH(CH3)2]5 and produced the ferroelectric tetragonal BaTiO3 nanoparticles at room temperature.17 Here, we report that the peptide assemblies from bolaamphiphile peptide monomers, (bis(N-α-amido-glycylglycine)-1,7-heptane dicarboxylate), have chemical moieties catalyzing kinetically unfavored crystal growth of monodisperse β-Ga2O3 nanoparticles via the aggregation-driven crystallization process (Figure 1). While the gallium precursors were hydrolyzed to GaOOH with base catalysts at room temperature, the gallium precursors were further hydrolyzed to β-Ga2O3 only when the peptide assemblies capped those particles. Unlike other growth methods, the crystallization with the peptide assemblies could grow single-crystalline and monodisperse β-Ga2O3 nanoparticles selectively from gallium precursors in high yield. This unique feature of the uniform and selective crystal growth would be regulated by the catalytic templating, the fusion of nucleated particles, and the nanoscale growth confinement in the peptide assemblies.
Figure 1.
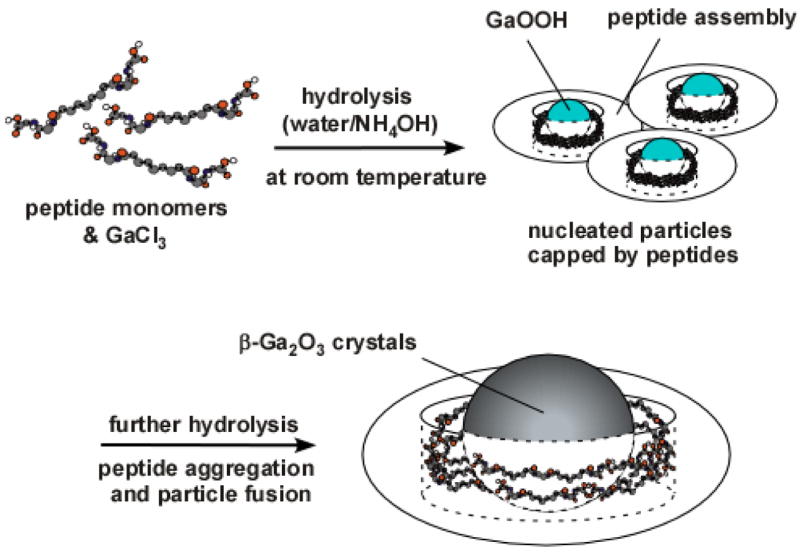
Illustration of β-Ga2O3 growth mechanism in the peptide capping assembly.
EXPERIMENTAL
Materials
The peptide assemblies were prepared by the self-assembly of the bolaamphiphile peptide monomers, bis(N-α-amidoglycylglycine)-1,7-heptane dicarboxylate. Detailed protocol for the preparation of bolaamphiphile peptide monomer is described in previous reports.18, 19 The gallium precursor, gallium(III) chloride (anhydrous, 99.99%), and ammonia solutions (29.2% and 5.0M, respectively) were obtained from Sigma-Aldrich and used as received.
Hydrolysis of gallium precursors in peptide assemblies
When 1 ml of bolaamphiphile peptide monomer solution (4.2 mg/ml) was mixed with 100 μl of GaCl3 solution (10 mM) under nitrogen and then a volume of 200 μl of NH4OH solution (15 M) was added to above mixture to adjust its pH as 10.0, the gallium precursors were hydrolyzed to GaOOH capped with the peptide assemblies after one week. After two weeks of the hydrolysis in dark at room temperature, both GaOOH and β-Ga2O3 nanoparticles associated with the peptide assemblies were observed. After three weeks, all GaOOH nanoparticles were converted to β-Ga2O3 nanoparticles in the peptide assemblies in various particle sizes. After four weeks, all particles in the peptide assemblies were grown to β-Ga2O3 nanoparticles in the diameter of 50 nm. As a control experiment, the same hydrolysis of gallium precursor was examined at pH 7 by balancing acidity with 500 μl of citric acid (0.5 M), and only GaOOH nanocrystals was formed in this condition. Another control experiment was examined under the same experimental condition without the peptide and this condition also yielded only GaOOH particles.
Characterization
The shape and crystalline structures of synthesized nanocrystals were studied using transmission electron microscope (TEM, JEOL Model 1200EX, 100ekV) and accompanying selected area electron diffraction (SAED). The SAED patterns were obtained at the camera distance of 12 cm or 60 cm. In the preparation of the TEM specimens, a volume of 9 μl of the nanocrystal suspension was dropped on the fomvar/carbon-coated copper grid and dried in the air. No staining solution was applied to prevent artifacts.
RESULTS AND DISCUSSION
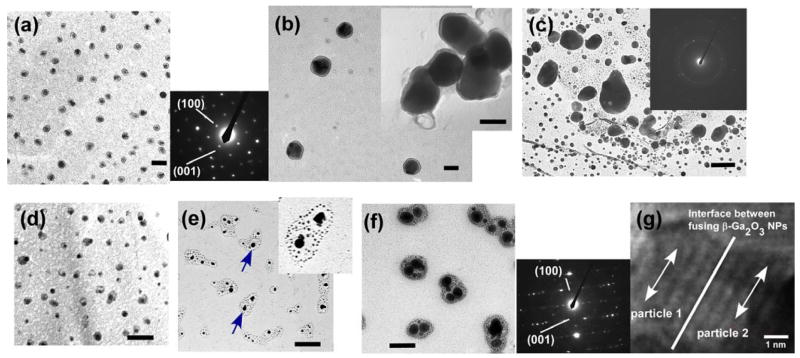
After the bolaamphiphile peptide monomers were associated with the gallium precursors for one month at pH 10, β-Ga2O3 nanoparticles were synthesized and the TEM image is given in Figure 2-a. This TEM image shows that those nanoparticles were grown in an average diameter of 50 nm with a narrow size distribution (supporting information), and their selected area electron diffraction (SAED) pattern (inset of Figure 2-a) shows the single crystalline structure of monoclinic form of β-Ga2O3. This nanoparticle had an emission at 389 nm, consistent with photoluminescence (PL) of β-Ga2O3 nanoparticles (supporting information).4 Magnified TEM images in Figure 2-b show layers of peptides around the core particles. When the gallium precursor was hydrolyzed at the same experimental condition without the peptide, polydisperse particles in the diameters of 15 – 150 nm were observed as shown in Figure 2-c, and their SAED shows that these particles were GaOOH (inset of Figure 2-c). The base solution hydrolyzes gallium precursors to form GaOOH, however it is not strong enough to drive the hydrolysis further to form β-Ga2O3 through the condensation.6 Therefore, these outcomes indicate that the peptides have the catalytic function to grow β-Ga2O3 nanoparticles at room temperature.
Figure 2.
TEM image of β-Ga2O3 nanoparticles after four weeks of hydrolysis with the peptide (a) in low magnification and the SAED pattern. Scale bar = 100 nm. (b) in medium magnification (inset: high magnification). Scale bar = 50 nm. (c) TEM image of GaOOH particles grown without the peptide and the SAED pattern. Scale bar = 80 nm. (d) TEM image of GaOOH nanoparticles grown after one week of hydrolysis with peptides. Scale bar = 20 nm. (e) TEM image of nanoparticles grown with the peptide after two weeks (inset: high magnification). Scale bar = 50 nm. (f) TEM image and SAED pattern of β-Ga2O3 nanoparticles after three weeks of hydrolysis with peptides. Scale bar = 50nm. (g) HRTEM of fusing β-Ga2O3 nanoparticles. (200) lattice faces for both particles 1 and 2, shown by arrows, are aligned parallel to the particle-merging interface.
To understand the growth mechanism, the nanoparticle growth was monitored with TEM and SAED at different growth stages. After one-week hydrolysis of gallium precursor with the peptide, small nanoparticles in a diameter of ~10 nm were observed with the capping peptide assemblies (Figure 2-d). However, those particles were probed to be GaOOH from their SAED pattern. After two weeks of hydrolysis, both aggregated larger nanoparticles and smaller nanoparticles were observed in the peptide assemblies (Figure 2-e). Some of the gallium nanoparticles fused with each other to form larger particles, indicated by arrows. Smaller particles were also incorporated in the aggregated peptide assemblies shown in the inset of Figure 2-e. This fusion of particles inside the peptide assemblies was further proceeded after three weeks, as shown in Figure 2-f. Since those small particles and the fusing nanoparticles were not observed in the four-week old sample in Figure 2-a and the size of Ga2O3 nanoparticle in the four-week old sample was larger than the one in the two-week old sample, these results suggest that the small particles in Figures 2-e and 2-f fused each other to grow larger Ga2O3 nanoparticles in the peptide assemblies. This type of the nanoparticle fusion in capping agents to grow mesoscale crystals, aggregation-driven crystallization, was observed in various biomineralization processes in nature, and recently this process was mimicked to grow mesocrystals in organic media by fusing primary nanoparticle-building blocks in aggregated micelles and capping polymers.20–22 In the same fashion, it is plausible that the highly crystalline β-Ga2O3 particles in the diameter of 50 nm were formed by the fusion of small particles in the capping peptide assemblies. To obtain a better understanding of the particle-fusion mechanism, the SAED patterns of nanoparticles at different growth stages were compared. The SAED of fusing nanoparticles in the three-week old sample (Figure 2-f) shows the fainted and fused (100) and (001) spots. This pattern indicates that those particles preferentially oriented to their [010] crystallographic direction and their [100] and [001] directions were not aligned well.23 The SAED of the fused nanoparticles in the four-week old sample (Figure 2-a) shows the strong intensities of the (100) and the (001) spots with the single crystalline structure. These SAED patterns indicate that the nanoparticles in Figure 2-f were aggregated along the [010] direction to grow single-crystalline β-Ga2O3 particles shown in Figure 2-a.23 HRTEM of fusing β-Ga2O3 nanoparticles at the particle-merging interface also supports this growth mechanism. As shown in Figure 2-g, the (200) lattice fringe of the particles 1 was parallel to the (200) lattice fringe of the particles 2 around the merging area. This HRTEM image indicates that β-Ga2O3 nanoparticles are fused to the preferential orientation of their [010] crystal direction, consistent with the SAED results.
Because β-Ga2O3 nanoparticles were observed with the existence of peptide after two weeks of hydrolysis and only GaOOH particles were obtained in the same experimental condition without the peptide, the peptide assemblies must have a catalytic activity in the hydrolysis reaction of gallium precursors. Based on the study on proteins of glassy skeletal spicules in marine sponge, silicateins, it has been shown that chemical moieties of nucleophilic hydroxyl groups of serine catalyzed hydrolysis reactions when they were hydrogen-bonded with amine groups of the neighboring histidine.6 Similarly, the carboxyl group in the peptide assemblies could also catalyze the hydrolysis as they are hydrogen-bonded with the neighboring amine. Although the carboxyl moiety is a weaker hydrolysis agent comparing to hydroxyl,24 the hydrogen bonding with the amine could amplify nucleophilicity of the carboxyl group to strengthen the catalytic activity of the hydrolysis, as observed in the silicatein. To probe the complexation among the carboxyl, the amine, and gallium ions, FT-IR spectroscopy was applied. FT-IR spectrum of the gallium-peptide complexes in black line in Figure 3 shows characteristic symmetric COO−, asymmetric COO−, and bands of the bridged metal complex, COO− -Ga-NH2+1423 cm−1, 1563 cm−1, and 1620 cm−1, respectively, in addition to peaks originated from the peptide assemblies.25, 26 This observation supports our hypothesis that the gallium hydrolysis took place at the juxtaposition of the carboxyl and the amine moieties in the peptide assembly.
Figure 3.
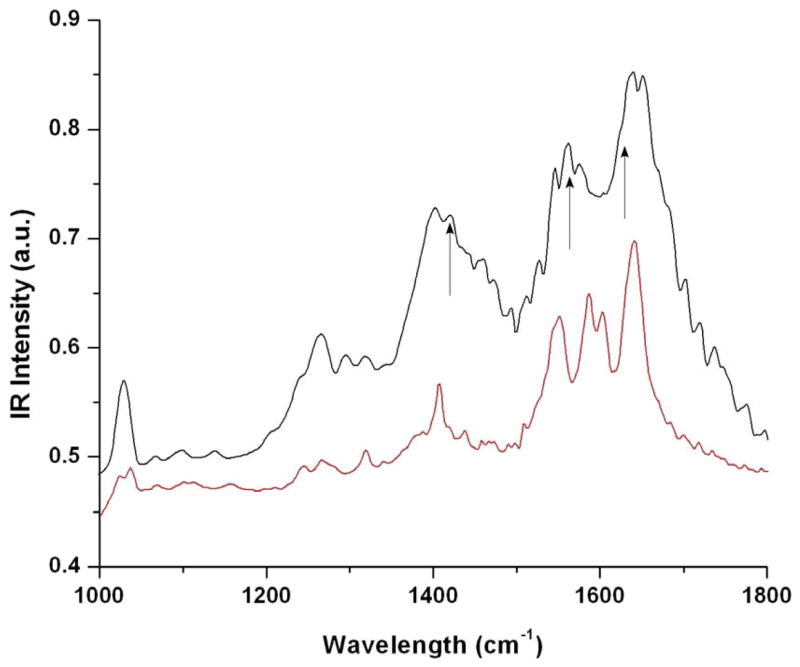
FTIR spectra of neat peptides (red) and peptides complexed with the gallium ions (black). Arrows in (b) show peaks corresponding to vibrations of COOH and NH2 complexing with gallium ions.
To confirm our hypothesis that the hydrolysis is catalyzed by hydrogen-bonded carboxyl groups of the peptide, we examined a control experiment to weaken the strength of hydrogen bonds between the carboxyl and the amine groups during the growth process. When the gallium precursor was hydrolyzed at pH 7, the protonated carboxyl group bound the amine group weaker and the weaker nucleophilicity of the carboxyl reduced the degree of the gallium hydrolysis. Under this condition, after the hydrolysis of the gallium precursor with the peptide for four weeks, only GaOOH crystals were observed in the peptide assemblies due to the lack of strong hydrolysis activity (Figure 4-a). This result is consistent with our hypothesis that the chemical moieties of hydrogen-bonded carboxyl and amine catalyze the hydrolysis of gallium precursors to form β-Ga2O3 crystals.
Figure 4.
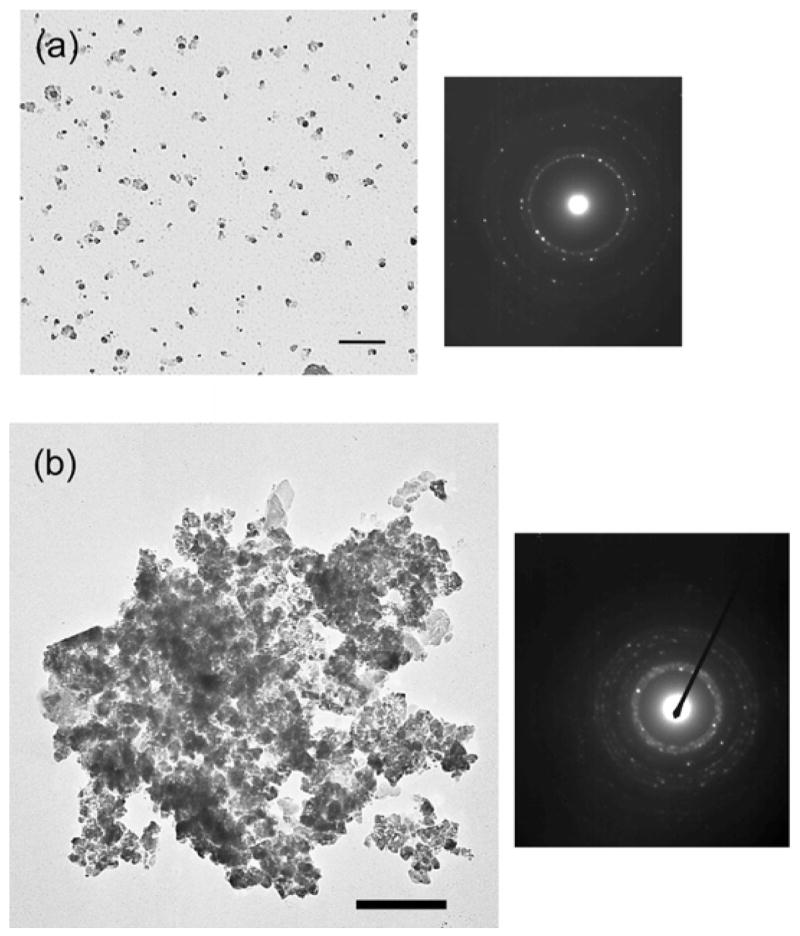
TEM images and electron diffractions of (a) GaOOH particles obtained after four weeks of hydrolysis with the peptide at neutral pH. Scale bar = 100 nm. (b) GaN particles obtained after the sintering of Ga2O3 nanoparticles under NH3 at 900 °C. Scale bar = 200 nm.
In addition to the influence of chemical moieties of peptide for the catalytic activity, the shape and the dimension of capping peptide assembly could also promote the gallium hydrolysis. Previously, the dehydration of calcium carbonate was accelerated in porous templates because those pores functioned as pathways for the water exclusion and the dehydration.7 The pores of the peptide assemblies capping nanoparticles could also provide the dehydration pathway promoting the β-Ga2O3 growth in the cavities. Another potential factor to contribute the β-Ga2O3 crystallization in the peptide assembly is the high surface tension built in the nanoscale peptide cavities. Previously, when mineralization of calcite occurred in micron-scale pores of membranes, the resulting crystals in the pores were observed to have unusual crystalline structures due to high surface tensions in such small confinements that altered the reaction kinetics of the crystal growth.27 We believe that the peptide templates could also exploit this strategy of the confinement-controlled mineralization one step further to nanoscale and the confinement effect of the peptide assemblies could assist the unusual β-Ga2O3 crystallization at room temperature.
When these nanoparticles were sintered at 900 °C under NH3, the peptide assemblies were removed and the oxide particles were transformed to GaN (Figure 4-b). Through the sintering process, the peptide assemblies were removed. The baked GaN nanoparticles were aggregated, and this aggregation is probably due to the high surface energy of uncapped nanoparticles and the sample drying effect
CONCLUSIONS
Peptide assemblies were applied as catalytic nanoreactors to grow β-Ga2O3 at room temperature. This peptide assembly template accompanying with the enzymatic functionality catalyzed the hydrolysis of gallium precursors. While the conventional base catalyst hydrolyzed the gallium precursors to GaOOH, the peptide assembly could further promote the reaction to crystallize β-Ga2O3. Due to the catalytic chemical moieties of the peptide, the kinetically unfavored β-Ga2O3 crystal growth was achieved in ambient conditions when the nucleated particles were capped by the peptide assemblies. In addition to the enzymatic chemical moieties of the peptide assembly, the nanoscale cavity created by capping particles could play both the efficient dehydration pathway and the optimal surface tension control, which are advantageous for the β-Ga2O3 crystal growth. This multifunctional peptide assembly is expected to be applied for the syntheses of a variety of nanomaterials that are kinetically difficult to grow at room temperature.
Supplementary Material
Supporting Information Available: the size distribution of β-Ga2O3 nanoparticles, AFM image of β-Ga2O3 nanoparticles, and PL spectrum of β-Ga2O3.
Acknowledgments
This work was supported by the U.S. Department of Energy (DE-FG-02-01ER45935) and the National Science Foundation CARRER Award (ECS-0103430). Hunter College infrastructure is supported by the National Institutes of Health, the RCMI program (G12-RR-03037). S.L. thanks Prof. C. Rojo and Dr. J. Carvajal for the use of electrical furnace in Department of Materials Science at State University of New York, Stony Brook. We also thank to Prof. K. Fath and Dr. A. Tsiola at Core Facilities for Imaging, Cellular and Molecular Biology at Queens College, City University of New York for the use of transmission electron microscope.
References
- 1.Pan ZW, Dai ZR, Wang ZL. Science. 2001;291:1947–1949. doi: 10.1126/science.1058120. [DOI] [PubMed] [Google Scholar]
- 2.Yin M, Gu Y, Kuskovsky IL, Andelman T, Zhu Y, Neumark GF, O’Brien S. J Am Chem Soc. 2004;126:6206–6207. doi: 10.1021/ja031696+. [DOI] [PubMed] [Google Scholar]
- 3.Nogales E, Mendez B, Piqueras J. Appl Phys Lett. 2005;86:113112. [Google Scholar]
- 4.Chang KW, Wu JJ. Adv Mater. 2004;16:545–549. [Google Scholar]
- 5.Colfen H, Antonietti M. Angew Chem Int Ed. 2005;44:5576–5591. doi: 10.1002/anie.200500496. [DOI] [PubMed] [Google Scholar]
- 6.Kisailus D, Truong Q, Amemiya Y, Weaver JC, Morse DE. Proc Natl Acad Sci USA. 2006;103:5652–5657. doi: 10.1073/pnas.0508488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aizenberg J, Muller DA, Grazul JL, Hamann DR. Science. 2003;299:1205–1208. doi: 10.1126/science.1079204. [DOI] [PubMed] [Google Scholar]
- 8.Brott LL, Naik RR, Pikas DJ, Kirkpatrick SM, Tomlin DW, Whitlock PW, Clarson SJ, Stone MO. Nature. 2001;413:291–293. doi: 10.1038/35095031. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad G, Dickerson MB, Church BC, Cai Y, Jones SE, Naik RR, King JS, Summers CJ, Kroger N, Sandhage KH. Adv Mater. 2006;18:1759–1763. [Google Scholar]
- 10.Sewell SL, Wright DW. Chem Mater. 2006;18:3108–3113. [Google Scholar]
- 11.Reches M, Gazit E. Science. 2003;300:625. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 12.Whaley SR, English DS, Hu EL, Barbara PF, Belcher AM. Nature. 2000;405:665. doi: 10.1038/35015043. [DOI] [PubMed] [Google Scholar]
- 13.Sano K, Ajima K, Iwahori K, Yudasaka M, Iijima S, Yamashita I, Shiba K. Small. 2005;1:826–832. doi: 10.1002/smll.200500010. [DOI] [PubMed] [Google Scholar]
- 14.Sarikaya M, Tamerler C, Jen AKY, Schulten K. Nature Mater. 2003;2:577–585. doi: 10.1038/nmat964. [DOI] [PubMed] [Google Scholar]
- 15.Kisailus D, Choi JH, Weaver JC, Yang WJ, Morse DE. Adv Mater. 2005;17:314–317. [Google Scholar]
- 16.Djalali R, Samson J, Matsui H. J Am Chem Soc. 2004;126:7935–7939. doi: 10.1021/ja0319691. [DOI] [PubMed] [Google Scholar]
- 17.Nuraje N, Su K, Haboosheh A, Samson J, Manning EP, Yang NL, Matsui H. Adv Mater. 2006;18:807–811. doi: 10.1002/adma.200501340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogiso M, Ohnishi S, Yase K, Masuda M, Shimizu T. Langmuir. 1998;14:4978–4986. [Google Scholar]
- 19.Matsui H, Gologan B. J Phys Chem B. 2000;104:3383–3386. [Google Scholar]
- 20.Colfen H, Mann S. Angew Chem Int Ed. 2003;42:2350–2365. doi: 10.1002/anie.200200562. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Mann S. Langmuir. 2000;16:7088–7094. [Google Scholar]
- 22.Wohlrab S, Pinna N, Antonietti M, Colfen H. Chem Eur J. 2005;11:2903–2913. doi: 10.1002/chem.200400420. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Sun H, Shao X, Li D, Yu H, Han M. Adv Mater. 2005;17:42–47. [Google Scholar]
- 24.Nudelman F, Gotliv BA, Addadi L, Weiner S. J Struct Biol. 2006;153:176–187. doi: 10.1016/j.jsb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Dutta PK, Gallagher PK, Twu J. Chem Mater. 1993;5:1739–1743. [Google Scholar]
- 26.Onoa B, Moreno V. Transition Met Chem. 1998;23:485–490. [Google Scholar]
- 27.Loste E, Park RJ, Warren J, Meldrum FC. Adv Func Mater. 2004;14:1211–1220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: the size distribution of β-Ga2O3 nanoparticles, AFM image of β-Ga2O3 nanoparticles, and PL spectrum of β-Ga2O3.