Abstract
In observational studies, the presence of confounding can distort the true association between an exposure and a toxic effect outcome if the confounding variable is not controlled either in the study design or the analysis phase. While confounding is often assumed to occur in the same direction as the toxicant exposure, the relationship between the benefits and risks associated with fish and seafood consumption is a classic example of negative confounding: the exposure to methylmercury occurs from fish and seafood which are also associated with beneficial nutrients, thereby counteracting the signs of mercury toxicity. Mercury and nutrients may affect the same epidemiological outcomes, but most studies addressing one of them have ignored the potential negative confounding by the other. This article reviews the existing evidence of effects of both nutrient and contaminant intakes as predictors of neurodevelopmental and cardiovascular outcomes. Substantial underestimation of the effects of mercury toxicity and fish benefits occurs from the lack of confounder adjustment and imprecision of the exposure parameters. Given this inherent bias in observational studies, regulatory agencies should reconsider current dietary advisories to provide better guidance to consumers in making prudent choices to maintain a nutritious diet with seafood that is low in mercury concentrations. Attention should also be paid to the occurrence of negative confounding in other connections.
Keywords: Confounding factors, Diet, Environmental exposure, Fish oils, Methylmercury compounds, Seafood
I. Introduction
Confounding is potentially present in all observational studies. It refers to a situation in which an association between an exposure and an outcome is distorted because it is mixed with the effect of a third variable – the confounding variable or the confounder. The confounding variable is related to both the exposure and the outcome, and it is not an intermediate step in the causal pathway between the exposure and the outcome (Rothman and Greenland, 1998). The distortion introduced by a confounder can lead to an overestimation (positive confounding) or underestimation (negative confounding) of the true association depending on the direction of the effects the confounder has with regard to exposure and outcome. It is therefore important to anticipate and to control for the confounding variable to obtain a more precise estimate of the exposure effect.
Most attention is usually paid to confounders that affect the outcomes in the same direction as the exposure under study (positive confounding) (Blair et al., 2007), but the control for negative confounding is crucial in epidemiological studies of toxic exposures. The absence of proper adjustment will lead to underestimation of the toxicity of the exposure on the outcome and likewise the benefits of the confounder. A better assessment of the chemical toxicity will facilitate proper prevention programs.
The relationship between the benefits and risks associated with fish and seafood consumption is an important public health issue. On the one hand, fish and seafood provide an important pathway for human exposures due to the biomagnification of toxicants, such as methylmercury, in freshwater and marine food chains. Mercury contamination is now the main reason for fishing advisories in the United States (U.S. EPA, 2004). One the other hand, fish contains essential nutrients that may provide beneficial effects on brain development, and may prevent cardiovascular disease (CVD), thereby counteracting the adverse effects of methylmercury (Anon, The Madison Declaration on Mercury Pollution, 2007). This situation appears to constitute a clear example of negative confounding – the factors that affect the same outcome are associated as they derive from the same food items, i.e., fish and seafood (Figure 1).
Figure 1.
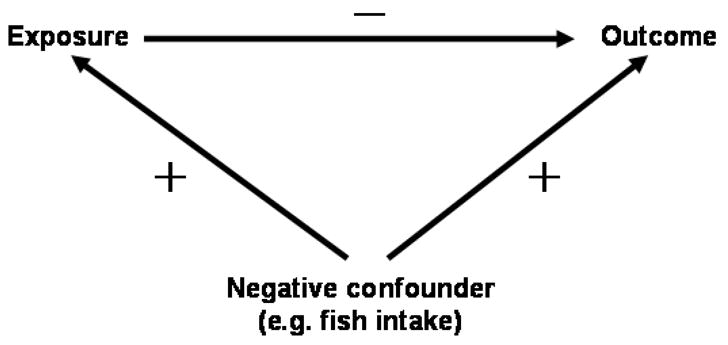
Negative confounding: fish intake (a negative confounder) is positively associated with both methylmercury (exposure) and neurodevelopment or cardiovascular health (outcome), and the exposure has adverse effects on the outcome. Failure to adjust for fish intake would result in underestimation of both mercury toxicity and fish benefits.
There is, however, considerable variability in mercury concentrations within and across species of dietary fish. Fish at low food chain levels have lower mercury concentrations. Similarly, there is considerable variability in the levels of long-chain polyunsaturated fatty acids (LCPUFA) across species. Fatty fish have higher levels of LCPUFA compared with lean fish, and freshwater fish largely have lower levels of LCPUFA compared with ocean fish (IOM/NAS, 2007; Mahaffey et al., 2004a).
This paper focuses on methylmercury in fish and seafood as a case study to discuss the effect of negative confounding on neurodevelopmental deficits in children and the risk of CVD in adults if fish intake is not properly adjusted for. Methylmercury toxicity will first be outlined, then the essential nutrients in fish and seafood that are crucial to the developing brain and prevention of heart diseases. The paper then reviews the epidemiological studies on mercury and fish nutrient interaction in regard to neurobehavioral and cardiovascular outcomes before it discusses the degree of underestimation of dose-effect relationship that results from exposure imprecision. When negative confounding is present, a substantial imprecision of the confounder – fish intake – can cause a further underestimation of the mercury effect even after confounder adjustment.
We focus on studies providing the strongest evidence and relevance in evaluating the association of methylmercury on neurodevelopment in children and the CVD risk in adults as a case study to discuss the effect of negative confounding if fish intake is not properly adjusted for. MEDLINE searches included combinations of “mercury” or “methylmercury”, “neurodevelopment” or “neurologic”, “cardiovascular” or “coronary”, “fish” or “fatty acids” or “omega-3”, or “fish oils”. We also used references cited in articles identified.
II. Methylmercury
Methylmercury is a worldwide contaminant found in seafood and freshwater fish. Toxic effects on the brain due to this organic mercury compound were first established in men with severe occupational exposure (Hunter and Russell, 1954). Evidence from poisoning outbreaks in Japan and Iraq clearly demonstrated the severe and widespread damage that may occur to the brain when exposed to methylmercury during development. In Minamata, Japan, infants were born with serious neurological damage, even if their exposed mothers were virtually unaffected (Harada, 1995; Igata, 1993).
Recent epidemiological studies have found more subtle adverse effects on brain functions at lower levels of methylmercury. Mercury-related neuropsychological dysfunctions were most pronounced in the domains of language, attention, and memory, and to a lesser extent, in visuospatial and motor functions; in addition, delayed latencies of the brainstem auditory evoked potentials (BAEP) were associated with both prenatal and recent methylmercury exposure (Debes et al., 2006; Grandjean et al., 1997; Murata et al., 2004). A recent case-control study found that an increased blood mercury concentration was associated with attention-deficit hyperactivity disorder (Cheuk and Wong, 2006).
Adverse effects of methylmercury exposure have also been associated with cardiovascular disease in adults. Epidemiological findings from Finland first showed that increases in mercury content in hair was associated with a progression of atherosclerosis and risk of cardiovascular diseases (Salonen et al., 1995, 2000; Virtanen et al., 2005). Because of the beneficial effects of fish and seafood nutrients, these outcomes may therefore also be affected by negative confounding.
A. Nutrients in fish and seafood
Certain essential nutrients in fish and seafood may provide beneficial effects on brain development, and may protect against the development of heart disease, thereby possibly counteracting adverse effects of the toxicants. This counteraction could either involve a toxicokinetic interaction with methylmercury, but more likely the nutrients have an independent effect on the same outcomes as the food contaminant but in the opposite direction.
1. Polyunsaturated fatty acids
Fish and seafood are rich in long-chain n-3 polyunsaturated fatty acids (LCPUFA), mainly eicosapantaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) (Hearn et al., 1987; Raper et al., 1992), which are essential for normal brain development (Innis 1991). The LCPUFA control the composition of membranes, hence their fluidity, and consequently, the enzymatic activities, the binding between molecules and receptors, the cellular interactions, and the transport of nutrients (Bourre et al., 1989). The growth of the human brain is the fastest from the beginning of the third trimester of gestation until about 18 months after birth (Innis, 1991), during which the demand for LCPUFA is the greatest. Insufficient supplies of LCPUFA and other nutrients during this critical period may result in deficits in brain development (Innis, 1991).
The LCPUFA in fish have also been suggested to play a key role in protecting against cardiovascular diseases (Doleck and Grandits, 1991; Kromhout et al., 1985). Possible key features include prevention of ventricular arrhythmias, antithrombotic, inhibition of the synthesis of cytokines and mitogens, and atherosclerosis (Connor, 2000).
2. Selenium
Selenium is a trace mineral that is essential to health. Fish and seafood, as well as eggs, meat, and vegetables are good sources of selenium. Selenium is a constituent of selenoproteins, which are important antioxidant enzymes and catalysts for the production of thyroid hormone (Rayman, 2000). Although the physiologic functions of selenium in the brain are not well understood, studies have found that selenium and certain selenoproteins are particularly well maintained despite prolonged selenium deficiency, suggesting an important role of selenium in this organ (Chen and Berry, 2003; Whanger, 2001). Low selenium levels have been reported to associate with accelerated progression of carotid atherosclerosis (Salonen et al., 1991), but other studies have not shown a clear relationship (Rayman, 2000).
Although methylmercury and selenium concentrations may be associated, selenium concentrations in ocean fish seem not to depend on fish size, while mercury concentrations increase with size (Kjellström, 2000). Intake and content of selenium vary considerably between countries and regions of countries largely due to the differences in geography, agronomic practices, food availability and preferences (Combs, 2001; Rayman, 2008; WHO, 1987). In China where toxic and deficient levels of selenium occur in different areas, an association between methylmercury and selenium levels was found in rice, but not in hair or blood samples (Horvat et al., 2003). Positive associations of methylmercury and selenium have also been reported among pregnant mothers and fish consumers in Sweden, where the selenium intake is marginally adequate (Ask et al., 2002; Björnberg et al,. 2003; Svensson et al., 1992). A moderate selenium and methylmercury association was observed in cord blood among the infants in Northern Quebec Inuit, where selenium intake levels are high (Muckle et al. 2001).
Although selenium has been considered to potentially provide protection against mercury effects, cord-blood selenium concentrations in the Faroe Islands did not impact on mercury-associated neurobehavioral deficits (Choi et al., 2008; Steuerwald et al., 2000).
3. Other nutrients
Among other nutrients, which may be supplied in part by seafood, iron is an essential component of proteins involved in oxygen transport, and is also essential for the regulation of cell growth and differentiation (Andrews, 2000). Iron deficiency may have a direct effect on the central nervous system, impacting brain growth, neurotransmitter levels, as well as toxicity or intracellular deficiency (Pollitt, 1993). Studies have found that iron deficiency has adverse effects on the cognitive and psychomotor development of children (Akman et al., 2004; Lozoff et al., 1987). Associations between iron status and coronary heart disease have been inconsistent. High iron stores have been linked with increased risk of coronary heart disease in some studies (Salonen et al., 1992; Sempos et al., 1994), but not others (Danesh and Appleby, 1999; Ma and Stampfer, 2002).
Iodine, a trace element, is an essential component of thyroid hormones, which are required for normal development and metabolism. Fish and seafood are rich sources of iodine. Iodine deficiency is the leading cause worldwide of preventable mental retardation and brain damage (ICCIDD, 2005). The central nervous system development depends on an adequate supply of thyroid hormone, which requires iodine for biosynthesis (Maberly, 1994a). Infants and children with iodine deficiency are at risk for poor mental and psychomotor development (Bleichrodt and Born, 1994; Morreale et al., 1989). The disorders increase with the extent of deficiency, with overt endemic cretinism as the severest consequence, resulting in irreversible mental retardation, neurological damage, and thyroid failure (Delange, 2000).
Vitamin E may interact with selenium additively because of their similar antioxidant roles (Maberly et al., 1994b). Fish is a source of Vitamin E, although vegetable oils are the most abundant sources. There is evidence to suggest that deficiency in Vitamin E may be associated with neurological functions in children and adults (Kalra et al., 1998; Sokol, 1989).
B. Mercury effects and fish nutrient interactions in epidemiological studies
From the evidence available, methylmercury exposure may adversely affect the neurobehavioral development in children and may promote cardiovascular diseases. However, nutrients in fish and seafood may affect the very same outcomes, although in the opposite direction. Without addressing the negative confounding in the epidemiological study design or data analysis, an underestimation of the effect will occur both of the methylmercury exposure and of the nutrient intake, depending on which of the risk indicators that is chosen as the focus of the study. Unfortunately, the great majority of cohort studies in this field has focused either on the risk of methylmercury or on nutrient benefits, but not both. We therefore outline the different health outcomes and highlight the small number of studies that have aimed at examining the effects of both nutrient and methylmercury exposure at the same time as predictors of developmental and cardiovascular disease outcomes.
1. Major prospective cohort studies on methylmercury exposure
Three major prospective cohort studies of methymercury-exposed children have been conducted in New Zealand, the Faroe Islands, and the Seychelles. Table 1 outlines the major differences between these three prospective studies.
Table 1.
Main differences between three major prospective studies of methylmercury-exposed children
| Attribute | New Zealand | Faroes | Seychelles |
|---|---|---|---|
| Source of exposure | Shark and ocean fish | Whale, ocean fish and shellfish | Ocean fish |
| Mercury exposure assessment | Maternal hair | Cord blood, cord tissue, and maternal hair | Maternal hair |
| Mercury effect | Significant | Significant | Not significant |
| Effect of maternal fish intake | Mothers were matched for high fish intake | Adjustment for maternal fish intake increased mercury effect | Maternal fish intake not included in data analysis |
| Other toxicant exposures | Lead in house paint and air | PCBs (whale blubber) | Tropical pesticide use |
| Language | English (and Pacific languages) | Faroese (and Danish) | Creole (English and French) |
| Socioeconomic setting | Industrialized Western | Industrialized Scandinavian | Middle-income developing |
| Family-setting | Urban, mixed cultures | Traditional | Mainly matriarchal |
| Outcome tests | Omnibus | Domain-related and neurophysiological | Omnibus and domain -related |
| Clinical examiners | Clinical specialists | Clinical specialists | Nurse/student |
a. New Zealand
A cohort of 11,000 mothers, who gave birth to children in 1978, was initially screened (Kjellström et al., 1986). Hair mercury concentrations were determined for 1000 mothers, who had consumed 3 fish meals per week during pregnancy. Seventy-three mothers (Pacific Island descent, 62%; Maoris, 27%; and Europeans, 11%) had a hair mercury level above 6 μg/g, thereby constituting the high-exposure group. At the first follow-up at age 4 years, 31 high-exposure children and 31 reference children with lower exposure were matched for potential confounders (i.e., mother’s ethnic group, age, child’s birthplace, and birth date). The high-exposure group showed significant lower scores on the Denver Developmental Screening test, a standardized test for childhood mental and motor development.
A follow-up of the original cohort was carried out at age 6 years. The 61 high-exposure children were matched with three control groups: one group with maternal hair mercury levels of 3–6 μg/g, and two groups with levels below 3 μg/g (one group with high fish consumption and an additional one with low fish consumption). Matching parameters included ethnic group, age, smoking habits, residence, and sex of the child. Psychological performance tests, which included the Wechsler Intelligence Scale for Children (WISC-R), the McCarthy scales for children’s abilities (perceptual and motoric) and the Test of Oral Language Development (a standardized test used in child development studies in New Zealand) were strongly associated with the maternal hair mercury concentration (Kjellström et al., 1989). This study included matching for the number of fish dinners during pregnancy and therefore may not require further adjustment for negative confounding.
b. Faroe Islands
The Faroe Islands are located in the North Atlantic between Norway, Shetland, and Iceland. Excess exposure to methylmercury is mainly due to the traditional habit of eating meat from the pilot whale in this fishing community. Fish intake varied but shows a positive association with whale intake. Ingestion of whale blubber causes exposure to lipophilic contaminants, notably polychlorinated biphenyls (PCBs). The first birth cohort consisted of 1,022 children born during a 21-month period in 1986–1987 (Grandjean et al., 1997). Prenatal methylmercury exposure was determined from mercury concentrations in cord blood and maternal hair, both spanning a range of about 1000-fold. A total of 917 eligible children (90.3%) participated in the detailed examination at school age (7 years). The physical examination included a sensory function assessment and a functional neurological examination with emphasis on motor coordination and perceptual-motor performance. Main emphasis was placed on detailed neurophysiological and neuropsychological function tests that had been selected as sensitive indicators of abnormalities thought to be caused by methylmercury. A repeat examination was carried out at age 14 years, again with a high participation rate. The clinical test battery was similar to the one at 7 years.
At the 7-year and 14-year follow-up examinations, decrements in attention, language, verbal memory, and to a lesser extent, in motor speed and visuospatial function were associated with prenatal methylmercury exposure (controlled for age, sex, and confounders). Delayed latencies of the brainstem auditory evoked potentials (BAEP), and decreased heart rate variability were also associated with mercury exposure (Debes et al., 2006; Grandjean et al., 1997; Murata et al., 2004).
Another prospective study (cohort 2) of 182 singleton term births were examined by the Neurological Optimality Score (NOS) at age 2 weeks. Detailed information was obtained on exposures both to methylmercury and to lipophilic pollutants. The NOS showed significant decreases at higher cord-blood mercury concentrations, but neither with PCB nor with LCPUFAs (Steuerwald et al. 2000).
c. Seychelles
A pilot cohort study and a main study, each with approximately 800 mother-child pairs, were conducted in the Seychelles, an archipelago in the Indian Ocean (Shamlaye et al., 1995). A hair sample was obtained from the mother six months after parturition. The hair segment that represented the pregnancy period was identified from the assumption that hair grows 1.1 cm per month. A subset of 217 children from the pilot cohort was evaluated at 66 months (Myers et al., 1995). Maternal hair mercury was negatively associated with the McCarthy General Cognitive Index, Perceptual Performance subscale, the Preschool Language Scale Total Language, and Auditory Comprehension subscale. After the authors had excluded apparent outliers, only auditory comprehension remained significant.
The main study included evaluation of the children at 6.5, 19, 29, and 66 months of age, and again at 8 years. No clear association with maternal hair mercury was found for most endpoints in these children (Myers et al., 1995; 2003). At 29 months there was an association between mercury exposure and decreased activity level in boys only, who also showed a possible mercury-associated delay in age for walking, but the latter was not significant when adjusted for confounders, which did not include fish intake. However, adjustment included the postnatal methylmercury exposure (Myers et al., 2003), but the extent to which this adjustment affected the findings is unclear.
A new longitudinal study of 300 Seychellois mother-child pairs was undertaken to assess the relationship between maternal mercury exposure and measures of LCPUFA with neurodevelopment in the children (Myers et al., 2007; Strain et al., 2007). Psychomotor development at 9 months of age increased with increasing maternal serum LCPUFA concentration and decreased with increasing maternal hair methylmercury concentration at 30 months of age. The adverse associations with methylmercury appeared only when nutrient status was added as a covariate, whereas the positive associations of developmental tests with LCPUFA likewise became much stronger after adjustment for methylmercury. LCPUFA data are not available from the previous Seychelles cohorts.
2. Neurodevelopmental outcomes
Tools used for outcome measurement need to be sensitive, specific and as independent as possible from study-specific administration procedures and cultural environment. Most studies employed a battery of neurobehavioral tests, some of which appeared to be more sensitive to mercury neurotoxicity than others, possibly due to superior psychometric properties, not necessarily greater sensitivity to mercury. Test results summarized as regression coefficients expressed as a proportion of the standard deviation, or as a delay in mental development calculated from the regression coefficient for age may provide guidance for identifying the most sensitive parameter. Benchmark dose levels may also be used as a basis for comparison. Thus, the most sensitive neurological, neuropsychological, and neurophysiological effect parameters all exhibit benchmark dose levels of 5–10 μg/g hair (NRC, 2000; Murata et al., 2004). Despite the great variability of the study settings and the outcome variables, a substantial degree of concordance exists and that the combined evidence is quite convincing in regard to the dose-response relationship.
a. Neurological tests
Neurological examination has been included in prospective studies (Steuerwald et al., 2000) and cross-sectional studies (Cordier et al., 2002; Marsh et al., 1995). The clinical tests, however, provide only suggestive evidence linking low-dose methylmercury exposures to detectable abnormalities. The absence of clear, positive findings most probably reflects the lack of sensitivity of this type of examination within this range of exposures and the potential imprecision in assessment due to the subjectivity of the evaluation.
b. Neuropsychological tests
While likely to be more sensitive in revealing early neurotoxic changes, neuropsychological tests require that the administration is standardized, and they may show examiner-dependence. In addition, they may be sensitive to details in the test situation, such as the use of an interpreter, and other aspects that may be important when a test is used in a particular culture different from the one where it was originally intended for.
Traditionally, studies in this field have included standard intelligence batteries. For example, the Wechsler Intelligence Scale for Children (WISC) and the McCarthy Scale of Children’s Abilities were included in New Zealand (Kjellström et al., 1989) as well as the Seychelles (Davidson et al., 2001; Myers et al., 2003). These intelligence tests may not be the most appropriate and sensitive for methylmercury toxicity, although significant results were found on WISC and McCarthy in New Zealand. Some WISC subtests were administered by an interpreter, e.g. in Madeira (Murata et al., 1999b), thereby making the test results less reliable. The approach to neurobehavioral assessment taken by the Faroes was to emphasize tests that reflected functional domains (e.g. attention, motor speed, verbal memory) most likely to be affected by developmental methylmercury exposure, as judged from the location of neuropathological lesions in poisoning cases and as illustrated by studies of other developmental neurotoxicants, especially lead. The Boston Naming test (language) showed a wide range of responses and appeared to be the most sensitive and reliable outcome (NRC, 2000).
Similar methods have been used in studies of LCPUFA, which are transferred via the placenta or supplied in human milk as necessary nutrients for normal brain growth and development in infancy and also likely to play an important role in the development of infant cognition (Clandinin et al., 1989; Willatts and Forsyth, 2000). The assessment of cognitive benefits in children who were breastfed is hampered by the difficulty to disentangle the subtle effect of breastfeeding from its socioeconomic determinants (Zhou et al., 2007). Although the majority of studies conclude that breastfeeding promotes intelligence, the evidence from higher quality or more recent studies is less persuasive (Der et al., 2007; Jain et al., 2002). The role of LCPUFA is most clearly documented in randomized clinical trials.
Assessments on infant cognitive function included the Bayley MDI performance, the Fagan Test of Infant Intelligence, and the MacArthur Communicative Development Inventory (Willatts and Forsyth, 2000). Randomized studies on the effects of LCPUFA supplementation in infant formula have compared growth, measures of visual, motor, and mental development for both preterm and term infants (EFSA, 2005; Mahaffey, 2004a; Mozzaffarian and Rimm, 2006; Willatts and Forsyth, 2000). The results, however, have been inconsistent. For example, infants 18 months of age who were fed with formula supplemented with LCPUFA had higher Bayley MDI performance than the controls in one study (Birch et al., 2000), but not in another (Lucas et al., 1992). However, studies assessing the influence of LCPUFA on development of specific cognitive behaviors have shown a significant advantage for supplemented infants on measures of visual attention and problem solving, suggesting LCPUFA may enhance information processing or attention regulation in infants (Willatts and Forsyth, 2000).
c. Neurophysiological tests
As an objective means of evaluation of brain dysfunction likely to be less sensitive to motivation or socioeconomic confounding, neurophysiological tests have been applied in several studies. Their applicability requires advanced instrumentation and depends on skilled examiners. An outcome that has been previously found to be sensitive to lead exposure is brainstem auditory evoked potentials. Patients from Minamata, Japan, with congenital methylmercury poisoning exhibited delays in auditory brainstem evoked potential latencies (Hamada et al., 1982). The latency of peak III was significantly increased at higher intrauterine exposure to mercury. Parallel associations were found in 7-year-old children in the Faores (Murata et al., 1999a) and in Madeira (Murata et al., 1999b), and this observation was replicated in the Faroese cohort when examined at 14 years (Murata et al., 2004). As a parameter primarily affected by postnatal exposure, this particular outcome may provide unique insight in comparison with other functions sensitive to methylmercury during fetal development.
d. Visual function
Visual function and visual evoked potentials were not associated with mercury exposure in studies that did not account for possible beneficial effects of seafood nutrients (Grandjean et al., 1997, 2001). LCPUFA is particularly important for normal visual development. Thus, DHA normally accounts for greater than one-third of the total fatty acids of the brain gray matter (O’Brien et al., 1964; Crawford et al., 1976) and the retina of the eye (Anderson, 1970; Tinoco et al., 1977), and most of the prenatal accumulation of DHA in brain (Clandinin et al., 1980; Martinez, 1991) and retina (Martinez et al., 1988) in humans occurs in the last intrauterine trimester. Although DHA and the n-6 fatty acid arachidonic acid (AA) are the major structural components of the central nervous system, there is currently no consensus whether dietary supplementation of LCPUFA provides benefits for visual development of infants (Eilander et al., 2007). This evaluation considered effects of supplementation of pregnant and/or lactating women with DHA (no supporting evidence), effects of supplementation of preterm infants with DHA and AA on visual development at <6 months (inconclusive) and effects of supplementation of term infants at high doses on visual development during the first year of life (consistent evidence).
3. Nutrient and methylmercury exposure as predictors of developmental outcomes
Only a small number of studies have aimed at examining the effects of both nutrient and contaminant intakes at the same time as predictors of developmental outcomes. Three studies, in particular, showed that the effects of both nutrient and contaminant intakes were strengthened when both maternal fish intake and prenatal methylmercury exposure were included at the same time when modeling the outcomes (Oken et al., 2005; Budtz-Jørgensen et al., 2007; Myers et al., 2007; Strain et al., 2007). They will be reviewed later in this section. Among the other studies, a cohort of British children reported a beneficial association on developmental score with fish intake by mother during pregnancy and by the infant postnatally, while no clear effect of mercury concentrations in umbilical cord tissue (wet weight) was found (Daniels et al., 2004). However, the wet weight mercury concentration in the cord may be subject to some variability, thereby increasing the imprecision of the mercury exposure parameter at the low levels encountered.
In the smaller Faroese birth cohort 2, adverse neonatal neurological function was associated with increased prenatal methylmercury exposure, but neither n-3 fatty acids nor selenium provided any detectable beneficial or protective effect on this outcome (Steuerwald et al., 2000). No evidence that selenium was an important protective factor against mercury neurotoxicity was found in the Faroese birth cohorts 1 and 2 up to age 7 years (Choi et al., 2008).
In a Polish cohort of one-year old infants who were born to mothers exposed to low amounts of mercury during pregnancy, an association was found between cord blood and maternal blood mercury levels and delayed psychomotor development of infants. However, maternal fish intake was not included for adjustment in the analysis. Relationship between fish consumption in these mothers during pregnancy and mercury levels was assessed separately. In each trimester of pregnancy, fish intake was significantly associated with both maternal and cord blood mercury levels (Jedrychowski et al., 2006).
Three studies have found stronger effects of nutrient and contaminant intakes when both maternal fish consumption and prenatal methylmercury exposure were included in modeling the same neurodevelopmental outcomes (Oken et al., 2005; Budtz-Jørgensen et al., 2007; Strain et al., 2007):
Project Viva (Oken et al., 2005)
A small US study of neurodevelopment in infants suggested that maternal mercury exposure and fish intake has opposite effects on infant cognition. Table 2 shows the results that higher fish intake was associated with higher infant cognition after adjusting for participant characteristics, and the association was strengthened after adjustment for hair mercury. Both were statistically significant only in the joint regression equation. After adjustment for covariates including fish intake, an increase of 1ppm in maternal hair mercury was associated with a decrement in cognition score of 7.5 points (compared to 4.0 points without adjustment for fish consumption). Similarly, for each additional weekly fish serving consumed by mother, the child’s cognition score was 4.0 points higher (compared to 2.8 points without adjustment for maternal hair mercury). Similar results were found with the recent follow-up of the cohort at 3 years of age with stronger associations between higher fish intake and better child cognitive test performance, and higher mercury levels with poorer test scores when both fish and mercury were adjusted (Oken et al., 2008).
Table 2.
Association of maternal second-trimester fish consumption and maternal hair mercury at delivery with infant cognition at 6 months: results from six linear regression models adjusted for participant characteristics among 135 mother-infant pairs in Project Viva (Oken et al., 2005).
| Change in VRM score [% novelty preference (95%CI)] | ||
|---|---|---|
| Model | Effect per weekly fish serving | Effect per ppm maternal hair mercury |
| Fish only | 2.5 (−0.01 to 5.0) | – |
| Fish and participant characteristics | 2.8 (0.2 to 5.4) | – |
| Mercury only | – | −4.6 (−10.3 to 1.1) |
| Mercury and participant characteristics | _ | −4.0 (−10.0 to 2.0) |
| Fish and mercury | 3.9 (1.2 to 6.5) | −8.1 (−14.1 to −2.0) |
| Fish, mercury, and participant characteristics | 4.0 (1.3 to 6.7) | −7.5 (−13.7 to −1.2) |
Faroe Islands (Budtz-Jørgensen et al., 2007)
Adjustment for the benefits conferred by maternal fish intake during pregnancy resulted in an increased effect of the prenatal methylmercury exposure as compared to the unadjusted results in an analysis of structural equation modeling of the first Faroese birth cohort. Table 3 shows the previously reported mercury regression coefficients changed toward a larger mercury effect. The p-values for the mercury effect also decreased. For example, after adjustment for fish intake, the adverse mercury effect on motor function was 12.2 (p-value=0.0092) (compared to 9.74, p-value=0.034 without adjustment for fish intake) at 7 years, and 9.37 (p-value=0.0082) (compared to 7.41, p-value=0.033 with no fish adjustment) at 14 years. Fish intake had a beneficial effect on all the outcome functions considered, but was statistically significant only for the motor functions at 7 and 14 years of age, and spatial function at 14 years. The relatively limited impact on the mercury effect caused by adjustment for fish intake is probably due to the fact that the main source of methylmercury is pilot whale meat, and the association between mercury concentrations and frequency of fish dinners is weak (r = 0.25).
Table 3.
Mercury effects on neurobehavioral tests at 7 and 14 years of age, as determined in structural equation analysis with covariate adjustment before and after addition of the frequency of maternal fish dinners during pregnancy (Budtz-Jørgensen et al., 2007).
| Mutual adjustment
|
||||||
|---|---|---|---|---|---|---|
| Mercury without adjustment for fish intake
|
Fish intake
|
Mercury
|
||||
| Age/test group | Effect | p-Vaue | Effect | p-Value | Effect | p-value |
| 7 years | ||||||
| Motor | −9.74 | 0.034 | 25.1 | 0.010 | −12.2 | 0.0092 |
| Verbal | −10.4 | 0.0018 | 3.62 | 0.61 | −10.8 | 0.0017 |
| 14 years | ||||||
| Motor | −7.41 | 0.033 | 19.9 | 0.006 | −9.37 | 0.0082 |
| Attention | −8.40 | 0.029 | 12.2 | 0.13 | −9.54 | 0.016 |
| Spatial | 2.60 | 0.50 | 17.3 | 0.031 | 1.04 | 0.79 |
| Verbal | −5.97 | 0.080 | 9.85 | 0.16 | −6.87 | 0.049 |
| Memory | −2.86 | 0.39 | 3.15 | 0.64 | −3.05 | 0.37 |
Seychelles (Myers et al., 2007)
A new Seychelles study found that at 30 months of age, the psychomotor development decreased with increasing maternal hair methylmercury concentration when nutrient status was adjusted, whereas the positive associations of developmental tests with LCPUFA became much stronger when methylmercury was adjusted. A similar effect on the previously published results (Strain et al., 2007) is likely.
4. Cardiovascular outcomes
Although the developing brain is considered the critical target organ in regard to methylmercury, recent evidence has suggested that mercury from fish and seafood may promote or predispose to the development of heart disease. Studies in this field are complicated by negative confounding, as several cohort studies have found an inverse relationship between fish consumption and LCPUFA and mortality from coronary heart disease, although not all studies were supportive of a cardioprotective effect of LCPUFA (EFSA, 2005; Hallgren et al., 2001; IOM/NAS, 2007; Mozaffarian and Rimm, 2006). While this evidence is yet inconclusive, it deserves attention because it suggests that a narrow definition of subpopulations of interest, i.e., pregnant women, might leave out other vulnerable groups. We suggest that the presence of unadjusted negative confounding may be a major reason that the evidence still appears inconclusive or equivocal.
The first studies of methylmercury-associated cardiovascular disease were carried out in Finland (Salonen et al., 1995). An important report showed that the intima-media thickness of the carotid arteries was apparently associated with mercury exposure from fish (Salonen et al., 2000). More recent information tends to support these findings. A later study of Finnish men reported that an increased mercury exposure was associated with increased risk of acute coronary events and cardiovascular mortality, and that mercury also seemed to attenuate the protective effects of fish on cardiovascular health (Virtanen et al., 2005). These results are shown in Table 4. A large multi-center study from Europe showed an increased risk of cardiovascular disease associated with toenail mercury concentrations, and that high mercury content may diminish the cardioprotective effect of fish intake (Guallar et al., 2002). In a U.S. study of health care workers, only a minimal risk was seen even with the adjustment of n-3 fatty acid intake from fish in the multivariate models. However, after the exclusion of dentists with high toenail-mercury concentrations likely due to amalgam exposures, the mercury-associated risk in other health professionals was similar to the one observed in the European study, although not statistically significant (Yoshizawa et al., 2002).
Table 4.
Relative Risk (RR) associated with serum fatty acids according to hair mercury levels (Rissanen et al., 2000; Virtanen et al., 2005)
| RR (acute coronary events)a DHA+DPA (quintiles) | RRb | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Acute coronary event | CVD death | CHD Death | |
| Low hair Hg | 0.85 | 0.50 | 0.48 | 0.41 | 0.33 | 0.69 | 0.59 | 0.43 |
| High hair Hg | 1.00 | 0.83 | 0.63 | 0.76 | 0.76 | 1.06 | 0.87 | 1.05 |
Rissanen et al. (2000). Respective quintiles (proportion (%) of serum DHA+DPA of all fatty acids) are: Q1: <2.38%, Q2: 2.38–2.73%, Q3: 2.74–3.07%, Q4: 3.08–3.58%, Q5: >3.58%; low hair hg (lowest two tertiles): 0–2 μg/g; high hair hg (highest tertile): >2 μg/g
Virtanen et al. (2005). RR associated with each percentage unit increase in DHA+DPA; low hair hg: <2.03 μg/g, high hair hg: >2.03 μg/g (CVD: cardiovascular disease; CHD: coronary heart disease)
A possible mechanism may be induction of lipid peroxidation. In this regard, it is interesting to note that while essential fatty acids from fish may reduce the risk of acute coronary events, a high mercury content in fish could attenuate this beneficial effect (Rissanen et al., 2000). For example, men in the two lowest thirds of hair mercury (0–2 μg/g) who were also in the highest fifth of serum fatty acids had a 67% reduced risk of acute coronary events compared to men in the highest third of hair mercury who were at the same time in the lowest fifth of the serum fatty acids (Table 4). The increased risk seems to occur at hair-mercury concentrations above 2 μg/g, i.e., only twice the level corresponding to the U.S.EPA Reference Dose (a daily intake level designed to be without a significant risk of adverse effects over a lifetime).
Regular consumption of fish oil supplements appears to reduce the risk of coronary artery disease in some observational studies (Saldeen et al. 2002; von Schacky et al. 1999) but not others (Sacks et al. 1995). Recent randomized controlled trials have shown that LCPUFA in fish oil or fish can play a role in secondary prevention of coronary heart disease (Harper and Jacobson, 2005; Kris-Etherton et al., 2002). For example, the Diet And Reinfarction Trial (DART) reported a reduction in all-cause mortality in male myocardial infarction survivors advised to increase their intake of oily fish (Burr et al., 1989). Another randomized, placebo-controlled study of Indian patients found significantly lower non-fatal myocardial events in the fish oil and mustard oil groups (Singh et al,. 1997). A Norwegian study, however, reported no benefit in myocardial infarction patients who were given fish oil capsules compared with placebo, possibly due to the high habitual fish consumption among the general population in the area, with fish oil supplementation conferring no additional benefit (Nilsen et al., 2001). Results of the GISSI trial, the largest prevention study, found that PUFA supplements, but not Vitamin E, significantly reduced the risk of total mortality, nonfatal myocardial infarction, and stroke (GISSI-Prevenzione Investigators, 1999). The relative risks of cardiovascular death and of sudden death were also significantly reduced. These benefits were apparent within four months of randomization. The available studies point to an antiarrhythmic effect as a beneficial mechanism of LCPUFA on coronary heart disease (Kris-Etherton et al., 2002).
While most observational studies categorized fish intake on the basis of questionnaires, the randomized controlled trials mostly used capsules with and without LCPUFA. Commercially available fish oil capsules contain 20% to 80% of EPA and DHA by weight (200–800 mg/g) and also variable amounts of PCBs and dioxins (Mozaffarian and Rimm, 2006). Data concerning the level of mercury in commercial fish oil supplements are limited; a recent study tested five popular brands of fish oil supplements and found that they contained little or no mercury (from non-detectable (<6 μg/L) to negligible (10–12 μg/L)) (Foran et al. 2003). Similar results were reported in an analysis of popular fish oil supplements (Schaller 2001). Other brands of fish oils may have more mercury, depending on the source of fish and the processing. The presence of contaminants in both fish and fish oil supplements suggests the presence of negative confounding (Grandjean, 2005c). Additional studies are needed to better assess both benefits and risks of fish oil supplements. A useful approach may be to apply LCPUFA produced by certain species of marine algae that can be cultured industrially to provide the “fish” oil (Wen and Chen, 2003) as a promising alternative and contaminant-free source (Arterburn et al. 2007). However, plasma LCPUFA concentrations after fish or fish oil capsule intakes showed that LCPUFA from fish were more effectively incorporated into plasma lipids than when administered as capsules (Visioli et al. 2003). These issues may be of particular relevance during early development. Thus, among the preterm infants in a randomized clinical trial of dietary LCPUFA, synthesis of AA and DHA was found to decrease with age and was dramatically lower in by 7 months of age (Carnielli et al., 2007), thereby suggesting that PCPUFA synthesis is far from trivial in early life.
C. Other confounding variables
Fish and seafood are a primary source of EPA and DHA (IOM, 2007), but eggs and chickens, although not particularly rich sources, may contribute to the EPA and DHA content if frequently consumed. Plant sources, such as walnuts and flaxseed oil, contain ALA that can be converted to EPA and DHA. However, humans do not convert EPA or DHA from ALA at rates high enough to reach recommended intake levels (Pawlosky et al. 2001).
Apart from the intake of fish and seafood, and other non-fish based LCPUFA sources, other confounding variables may be of importance. Socioeconomic conditions vary substantially between the three major prospective studies on the neurotoxicity of methylmercury – New Zealand (Kjellström et al., 1986, 1989), the Faroe Islands (Grandjean et al., 1997; Debes et al., 2006), the Seychelles (Myers et al., 2003; Davidson et al., 2006) – as well as other cross-sectional studies. Within each study, socioeconomic differences may not be unimportant and independent of mercury exposures and should then be included in the confounder adjustment. For example, high intakes of methylmercury have been reported in certain ethnic groups (Innis et al., 2006; Sweeney et al.; 2006) and in social strata with a high sushi intake (Hightower and Moore, 2003), and vulnerability to contaminant effects may differ between socioeconomic groups.
Potential confounding was taken into account in previous studies of methylmercury neurotoxicity. For example, in the follow-up of the New Zealand birth cohort at ages 4 and 6 years, children whose mother had high hair mercury concentrations and reference children with lower exposure were matched for potential confounders including mother’s ethnic group, age, child’s birthplace, and birth date. In addition, study and control children were stratified according to fish consumption of mothers during pregnancy (Kjellström et al., 1986, 1989). Significant decrements in test performance in the children prenatally exposed to increased doses of methylmercury were found.
Other potential confounders include the family and home environment, which are documented as important determinants of childhood development. For example, in the New Zealand study, low social class and non-English home language reduced the score on some tests, and more than 6 months of breastfeeding increased the score on some tests (Kjellström et al., 1989). These variables were accounted for in the analysis.
Exposure to other toxicants should also be considered. The Faroese, for example, are exposed to polychlorinated biphenyls (PCBs) from eating whale blubber (Grandjean et al., 1997). However, no important impact of PCB exposure on the neurotoxicity outcomes were shown from detailed analyses of the Faroes data (Budtz-Jørgensen, 1999; Grandjean et al., 2003a; Steuerwald et al., 2000). The relative importance of PCB and mercury was assessed in structural equation analysis taking into account the imprecision in both variables. The inclusion of PCB exposure attenuated the mercury effect, but mercury remained statistically significant, while PCB was far from that (Budtz-Jørgensen et al., 2002). In New Zealand and Seychelles, the ocean fish consumed is unlikely to be contaminated by PCB, and the same would be the case with freshwater fish in the Amazon Basin (Grandjean et al., 1999).
Among major reasons why a mercury effect might have been overestimated, an expert group pointed to possible association of mercury intake with exposure to other neurotoxic pollutant(s) and other types of residual confounding (NIEHS, 1998). The best protection against confounding problems is to select a study setting where such concerns are unlikely and, if relevant, may be adjusted for appropriately. Thus, a homogenous society with limited difference in socioeconomic and cultural factors should be preferred. Although the existence of residual confounding can never be fully excluded, “phantom” covariates should not be uncritically invoked to explain away biologically plausible associations between methylmercury exposure and adverse effects. Disproportionate attention is usually concentrated on confounders that affect the outcomes in the same direction as the exposure under study. However, the control for negative confounding (opposite effect of the exposure) can be crucial in epidemiological studies. The absence of proper adjustment will lead to underestimation of the toxicity of the exposure on the outcome and the benefits of the confounder. In the case of methylmercury and fish, the toxicity of mercury and the benefits of the nutrients in fish and seafood will both be underestimated.
D. Exposure Imprecision
Standard multiple regression methods are often used to control for confounding effects. Even in the absence of confounding, adjustment for such established predictors such as sex, age, and maternal intelligence are usually included to obtain a more precise estimate of the mercury effect. In general, the prudent approach would be to include all covariates that may be potential confounders. However, in situations where the exposure is measured with some degree of imprecision, this approach may result in biased estimates. Inclusion of such covariates, which are associated with the exposure but without any explanatory power in regard to the effect, will then increase the underestimation of the effect of the exposure of interest (Budtz-Jørgensen et al., 2003).
The purpose of an exposure assessment is to provide a correct measure of exposure in terms of the amount that has reached the toxicological target during the relevant time period. Because the validity depends on the degree to which the exposure biomarkers reflect the “true” exposure, they can be considered only proxy variables, which are always imprecise to some extent (Grandjean et al., 2005a). In addition, the degree of imprecision of the exposure data is usually unknown. This issue is important since exposure misclassification (i.e., errors in matching the exposure-based biomarker of dose to the observed effect) is likely to cause underestimation of the true effect of the exposure.
In prospective studies, samples for mercury analysis have included maternal hair, cord blood, and cord tissue. Scalp hair is the most frequently used sample for methylmercury exposure assessment (Grandjean et al., 2002). However, hair mercury concentration is subject to variability such as hair type, hair color, external contamination and leaching due to permanent hair treatments (Grandjean et al., 2002; Yamamoto and Suzuki, 1978; Yasutake et al., 2003). These factors might well account for the greater overall imprecision of this biomarker. The blood concentration of methylmercury is often considered the appropriate indicator of the absorbed dose and the amount systemically available, but this biomarker may also be subject to possible variation (Grandjean and Budtz-Jørgensen, 2007). The dry-weight-based mercury concentration of the cord tissue seems to be a more precise parameter than the level expressed on a wet-weight basis (Grandjean et al., 2005b).
In assessing exposure biomarker imprecision and providing proper adjustment for its consequences, Grandjean and Budtz-Jørgensen (2007) documented that cord blood is the best available indicator of prenatal methylmercury exposure. However, the results also suggested that even the best exposure biomarker may be much more imprecise than suggested by laboratory quality data.
Exposure imprecision and thus misclassification will generally be non-directional, thus leading to an underestimation of dose-effect relationships (Grandjean et al., 2003b). This problem may be exaggerated by potential confounders that are correlated with the exposure. Inclusion of these variables in a regression may further add to the bias toward the null hypothesis, even in the cases where the potential confounder has no independent effect on the outcome (Budtz-Jørgensen et al., 2003).
Maternal dietary questionnaires have also been used to obtain information for the frequency of fish and seafood consumption. If detailed data on nutrient absorption levels are absent in the dietary questionnaire, the crude dietary variable will have substantial imprecision, thereby limiting the power to identify the effect of the confounder – fish intake. This imprecision also causes an underestimation of the fish-adjusted mercury effect (Budtz-Jorgensen et al., 2007). The distribution of mercury (and other chemicals) and LCPUFA (and other nutrients) are not homogenous in species of fish and seafood. There is a potential problem when established categories of fish consumption do not reflect well on mercury exposure. A recent observational study found no support for the advice that women of childbearing age should avoid high-mercury fish and eat up to 340 g of fish and shellfish per week (Hibbeln et al., 2007). This conclusion might have been obscured by the use of an exposure variable reflecting LCPUFA contents rather than methylmercruy (Mahaffey and Schoeny, 2007).
III. Discussion
When exposure to a toxicant occurs from a food source that is also associated with essential or beneficial nutrients, as in the case of methylmercury in fish and seafood, negative confounding occurs, resulting in underestimation of both mercury toxicity and fish benefits. The present paper demonstrates the likely substantial importance of negative confounding in this connection.
This phenomenon may also play a role in other toxicity assessments. The balance between breastfeeding benefits versus lactational toxicant exposure is another example of negative confounding, and the effect of breastfeeding on intelligence of children has been equivocal. Some studies have shown beneficial effect of breastfeeding on neurodevelopment (Kramer et al., 2008; Lanting et al., 1998; Lucas et al., 1992; Rogan and Gladen, 1993), but environmental toxicants, particularly PCBs and dioxins, are transferred through breast milk from mother to the child and may cause adverse effects (Jacobson et al., 1984; Walkowiak et al., 2001). Without controlling for the beneficial aspects of breastfeeding, the toxicity of PCBs and dioxins on neurodevelopment would be underestimated, as a result of negative confounding. Likewise, the benefits of breastfeeding may appear less when the effect is quenched by contaminant exposures. Nitrate and vegetables in regard to cancer development is another example of negative confounding as vegetables intake may protect against certain types of cancer, but rucola and other leafy vegetables can contain large amounts of nitrate, a risk factor for the formation of nitrosamines, which are carcinogenic (EFSA, 2008). The issue of potential negative confounders is relevant in regard to a range of exposure sources and toxic hazards, as illustrated in Table 5. Proper adjustment of the negative confounding variables is deemed important in obtaining a more precise estimate of the dose-effect relationship.
Table 5.
Examples of potential negative confounders associated with corresponding exposure sources and toxic hazards
| Exposure sources | Toxic hazards | Potential negative confounders |
|---|---|---|
| Freshwater fish, seafood | Methylmercury, PCBs, dioxins | Fish oil, vitamins, trace elements |
| Fruits and nuts | Aflatoxin, pesticide residues | Vitamins, antioxidants |
| Vegetables | Nitrate | Anticarcinogens |
| Red meat | Beef hormones, contaminants | Complete proteins |
| Artificial sweeteners | Possible carcinogens | Caloric restriction |
| Breast-feeding | Maternal lipophilic drugs, pollutants | Essential nutrients, antibodies, psychological benefits |
| Occupation | Hazards at work | Benefits from being employed |
To clarify the confusion the public has on the relative risks and benefits of fish and seafood consumption, advisories against mercury contamination and recommendations on nutrient intakes must be reconciled. Unfortunately, the great majority of cohort studies in this field has focused either on the risk of methylmercury or on nutrient benefits. For example, the results of an observational cohort study reported beneficial effects on child development at maternal seafood intakes of more than 340 g per week, a recommended level for pregnant women or women likely to become pregnant (Hibbeln et al., 2007). However, the study focused on the nutrient benefits in fish only and failed to adjust for local seafood contaminants, such as methylmercury or PCBs; although the authors discussed the potential harmful effects of methylmercury. In a review, Mozaffarian and Rimm (2006) performed analyses of studies to evaluate methylmercury contamination and cardiovascular disease. A non-significant overall pooled relative risk of 1.12 was reported, but the meta-analysis included studies with dentists as participants, who were mainly exposed to inorganic mercury from amalgam, and not methylmercury from fish intake and therefore should have been excluded. However, the authors acknowledged that methylmercury may be a risk factor for cardiovascular disease but that potential risks and benefits of fish intake must be considered in context.
Among the small number of studies that examined the effects of both nutrient and methylmercury at the same time as predictors of developmental outcomes (Daniels et al., 2004; Oken et al., 2005; Steuerwald et al., 2000), the results were probably affected by the imprecision of the exposure parameters, which may bias the findings toward the null hypothesis and exaggerate the effects of confounding (Budtz-Jørgensen et al., 2003). Likewise with the small number of epidemiological studies of cardiovascular parameters that included both fatty acid intakes and mercury exposures (Guallar et al., 2002; Virtanen et al., 2005; Yoshizawa et al., 2002), the results most likely underestimate the true effect of methylmercury exposure because of misclassification of methylmercury exposure that was based on the mercury concentration in toenails or hair (Budtz-Jørgensen et al., 2007).
None of the three major prospective studies of developmental methylmercury neurotoxicity used for risk assessment incorporated adjustment for fish intake in the data analysis (NRC, 2000). The New Zealand study is likely affected to a lesser degree by confounding because the study design required matching of maternal high fish intake at different levels of methylmercury exposures (Kjellström et al., 1989). The confounding may be greater in the Seychelles as the average fish intake is high, and because the methylmercury exposure originates solely from fish (Clarkson and Strain, 2003). To compare the three studies, differences in exposure assessment, sources of bias, and the sensitivity of the outcome parameters to subclinical neurotoxicity must also be taken into account (Grandjean et al., 2005a). Despite the apparent differences among the populations, they may not necessarily be in disagreement. For example, when the maternal hair mercury concentration is selected as the exposure biomarker in both the Faroes and the Seychelles studies, and the Boston Naming Test is used as the outcome parameter, the confounder-adjusted regression coefficients from the two studies do not differ to a statistically significant extent (Keiding et al., 2003). The results would likely be more similar if the confounding effects of nutrients could be separated from the effects of methylmercury (Budtz-Jørgensen et al., 2007).
The negative confounding in the case of methylmercury in seafood and fish results from the effects of a related, but beneficial parameter – nutrients originating from the same source. While adjustment for the negative confounding is crucial, the imprecision of this confounder may by itself also cause substantial underestimation of the effects of methylmercury toxicity. Future studies should assess both beneficial and adverse effects of fish and seafood intake at the same time using reliable exposure parameters to separate the opposite impacts on the outcomes.
The distribution of LCPUFA and methylmercury concentrations in fish and seafood are not homogenous. There are species that have a high content of beneficial nutrients, but do not necessarily contain much mercury (Gochfeld and Burger, 2005; Levenson and Axelrad, 2006; Mahaffey, 2004a; IOM/NAS, 2007). Lower mercury concentrations are usually present in younger specimens, small species, lower in the food chain; the nutrient contents appear to be relatively independent of these factors. Several species of salmon, mackerel, and herring are high in LCPUFA, providing an average of ~1.5 g combined EPA and DHA/100 g tissue, but typically average <0.1 μg/g mercury (Mahaffey, 2004a). On the other hand, the four fish species that have been on the federal FDA and EPA joint advisory (swordfish, tilefish, shark, and king mackerel) provide only ~0.3 g combined EPA and DHA/100 g edible tissue, and average ≥1 μg/g mercury. These four species contain one-fifth the amount of the major LCPUFA and more than ten times as much mercury as do several species of salmon, mackerel, and herring (Mahaffey, 2004a).
Given the presence of negative confounding and the likely underestimation of the effects of both toxicants and nutrients, consumers should be well-advised to make prudent choices in maintaining a nutritious diet with seafood that is low in mercury. Regulatory agencies should develop risk communication strategies for balanced messages regarding nutrients and mercury to assist the consumers in making this choice (Mahaffey, 2004b), which now appears even more important than before, for two reasons. In the light of negative confounding, this need would seem to be greater, because methylmercury appears to be more toxic than previously thought, and because the benefits of fish and seafood nutrients may also have been underestimated, both due to negative confounding.
Acknowledgments
We are grateful for the helpful comments from Dr. Esben Budtz-Jørgensen and Dr. Tord Kjellström. This work was supported by the U.S. National Institute of Environmental Health Sciences (ES09797 and ES11681). The contents of this paper are solely the responsibility of the authors and do not represent the official views of the NIEHS, NIH, or any other funding agency.
References
- Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr. 2004;93:1391–6. [PubMed] [Google Scholar]
- Anderson RE. Lipids of ocular tissues. IV. A comparison of the phospholipids from the retina of six mammalian species. Exp Eye Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Disorders of iron metabolism. N Engl J Med. 2000;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- Anon. The Madison Declaration on Mercury Pollution. Ambio. 2007;36:62–65. [PubMed] [Google Scholar]
- Arterburn LM, Oken HA, Hoffman JP, Bailey-Hall E, Chung G, Rom D, Hamersley J, McCarthy D. Bioequivalence of docosahexaenoic acid from different algal oils in capsules and in a DHA-fortified food. Lipids. 2007;42:1011–1024. doi: 10.1007/s11745-007-3098-5. [DOI] [PubMed] [Google Scholar]
- Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110:523–526. doi: 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term babies. Dev Med Child Neurol. 2000;42:178–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- Björnberg KA, Vahter M, Petersson-Grawé K, Glynn A, Cnattingius S, Darnerud PO, Atuma S, Aune M, Becker W, Berglund M. Methyl mercury and inorganic mercury in Swedish pregnant women and in cord blood: influence of fish consumption. Environ Health Perspect. 2003;111:637–641. doi: 10.1289/ehp.111-1241457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Stewart P, Lubin JH, Forastiere F. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50:199–207. doi: 10.1002/ajim.20281. [DOI] [PubMed] [Google Scholar]
- Bleichrodt N, Born MP. A metaanalysis of research on iodine and its relationship to cognitive development. In: Stanbury JB, editor. The damaged brain of iodine deficiency. New York: Cognizant Communication; 1994. pp. 195–200. [Google Scholar]
- Bourre JM, Dumont O, Piciotti M, Pascal G, Durand G. Polyunsaturated fatty acids of the n-3 series and nervous system development. In: Galli C, Simopoulos AP, editors. Dietary ω3 and ω6 fatty acids, biological effects and nutritional essentiality. New York: Plenum Press; 1989. pp. 159–175. [Google Scholar]
- Budtz-Jørgensen E, Keiding N, Grandjean P, White RF, Weihe P. Methylmercury neurotoxicity independent of PCB exposure [letter] Environ Health Perspect. 1999;107:A236–237. doi: 10.1289/ehp.107-1566427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1:2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P, White RF. Consequences of exposure measurement error for confounder identification in environmental epidemiology. Stat Med. 2003;22:3089–3100. doi: 10.1002/sim.1541. [DOI] [PubMed] [Google Scholar]
- Budtz-Jørgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Carnielli VP, Simonato M, Verlato G, Luijendijk I, De Curtis M, Sauer PJJ, Cogo PE. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am J Clin Nutr. 2007;86:1323–1330. doi: 10.1093/ajcn/86.5.1323. [DOI] [PubMed] [Google Scholar]
- Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86(1):12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- Cheuk DK, Wong V. Attention-deficit hyperactivity disorder and blood mercury level: a case-control study in Chinese children. Neuropediatr. 2006;37:234–40. doi: 10.1055/s-2006-924577. [DOI] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jørgensen E, Jørgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P. Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res. 2008;107:45–52. doi: 10.1016/j.envres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Strain JJ. Nutritional factors may modify the toxic action of methylmercury in fish-eating populations. J Nutr. 2003;133:1539S–1543S. doi: 10.1093/jn/133.5.1539S. [DOI] [PubMed] [Google Scholar]
- Clandinin ME, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Human Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- Clandinin MT, Chappel JE, van Aerde JEE. Requirements of newborn infants for long chain polyunsaturated fatty acids. Acta Paediatr Scand. 1989;78(Suppl 351):63–71. doi: 10.1111/j.1651-2227.1989.tb11212.x. [DOI] [PubMed] [Google Scholar]
- Combs CF. Selenium in global food systems. Br J Nutr. 2001;85:517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(suppl):171S–5S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, Josse D, White R, Amiel-Tison C. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Casperd NM, Sinclair AJ. The long chain metabolites of linoleic and linolenic acids in liver and brain in herbivores. Comp Biochem Physiol. 1976;54B:395–401. doi: 10.1016/0305-0491(76)90264-9. [DOI] [PubMed] [Google Scholar]
- Danesh J, Appleby P. Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation. 1999;99:852–854. doi: 10.1161/01.cir.99.7.852. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Longnecker MP, Rowland AS, Golding J ALSPAC Study Team. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402. doi: 10.1097/01.ede.0000129514.46451.ce. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Kost J, Myers GJ, Cox C, Clarkson TW. Methylmercury and neurodevelopment: reanalysis of the Seychelles child development study outcomes at 66 months of age. JAMA. 2001;285:1291–1293. doi: 10.1001/jama.285.10.1291-a. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Wilding GE, Shamlaye CF, Huang LS, Cernichiari E, Sloane-Reeves J, Palumbo D, Clarkson TW. Methylmercury and neurodevelopment: longitudinal analysis of the Seychelles child development cohort. Neurotoxicol Teratol. 2006;28:529–535. doi: 10.1016/j.ntt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at 14 years. Neurotoxicol Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delange F. The role of iodine in brain development. Proc Nutr Soc. 2000;59:75–9. doi: 10.1017/s0029665100000094. [DOI] [PubMed] [Google Scholar]
- Der G, Batty GD, Deary J. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945. doi: 10.1136/bmj.38978.699583.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doleck TA, Grandits G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT). Health effects of omega-3 polyunsaturated fatty acids in seafoods. In: Simopoulos AP, Kifer RR, Martin RE, Barlow SD, editors. World Rev Nutr Diet. Vol. 66. Basel, Switzerland: S Karger; 1991. pp. 205–216. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) (Last updated: 11/07/2006) Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to the safety assessment of wild and farmed fish. [accessed 4/17/2007]; http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178620762697.htm.
- EFSA (European Food Safety Authority) (Publication date: 05/06/2008) Nitrate in vegetables –Scientific Opinion of the Panel on contaminants in the food chain. [accessed 6/5/08]; doi: 10.2903/j.efsa.2008.653. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178712852460.htm. [DOI] [PMC free article] [PubMed]
- Eilander A, Hundscheid DC, Osendarp SJ, Transler PL, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2007;76:189–203. doi: 10.1016/j.plefa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Foran SE, Flood JG, Lewandrowski KB. Measurement of mercury levels in concentrated over-the-counter fish oil preparations: Is fish oil healthier than fish? Arch Pathol Lab Med. 2003;127:1603–1605. doi: 10.5858/2003-127-1603-MOMLIC. [DOI] [PubMed] [Google Scholar]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a compositie benefit-risk by dose curve. Neurotoxicol. 2005;26:511–520. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White R, Nielsen A, Cleary D, de Oliveira Santos E. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect. 1999;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, White R, Sullivan K, Debes F, Murata K, Otto DA, Weihe P. Impact of contrast sensitivity performance on visually presented neurobehavioral tests in mercury-exposed children. Neurotoxicol Teratol. 2001;23:141–146. doi: 10.1016/s0892-0362(01)00134-9. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Jørgensen PJ, Weihe P. Validity of mercury exposure biomarkers. In: Wilson SH, Suk WA, editors. Biomarkers of environmentally associated disease. Boca Raton, FL: CRC Press/Lewis Publishers; 2002. pp. 235–247. [Google Scholar]
- Grandjean P, White RF, Weihe P, Jørgensen PJ. Neurotoxic risk caused by stable and variable exposure to methylmercury from seafood. Ambul Pediatr. 2003a;3:18–23. doi: 10.1367/1539-4409(2003)003<0018:nrcbsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, Keiding N, Weihe P. Underestimation of risk due to exposure misclassification. Eur J Oncol Suppl. 2003b;2:165–172. [Google Scholar]
- Grandjean P, Cordier S, Kjellström T, Weihe P, Budtz-Jørgensen E. Health effects and risk assessments. In: Pirrone N, Mahaffey KR, editors. Dynamics of mercury pollution on regional and global scales: atmospheric processes and human exposures around the world. Norwell, MA: Springer; 2005a. pp. 499–523. [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, Jørgensen PJ, Weihe P. Umbilical cord mercury concentration as biomarker of prenatal exposure to methylmercury. Environ Health Perspect. 2005b;113:905–908. doi: 10.1289/ehp.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P. Contaminants in fish oil. Am J Clin Nutr. 2005c;82:1354. doi: 10.1093/ajcn/82.6.1354. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, Jørgensen PJ, Weihe P. Total imprecision of exposure biomarkers: implications for calculating exposure limits. Am J Ind Med. 2007 doi: 10.1002/ajim.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, Van’t Veer P, Bode P, Aro A, Gómez-Aracena J, Kark JD, Riemersma RA, Martin-Moreno JM, Kok FJ Heavy Metals and Myocardial Infarction Study Group. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Hallgren CG, Hallmans G, Jansson JH, Marklund SL, Huhtasaari F, Schütz A, Strömberg U, Vessby B, Skerfving S. Markers of high fish intake are associated with decreased risk of a first myocardial infarction. Br J Nutr. 2001;86:397–404. doi: 10.1079/bjn2001415. [DOI] [PubMed] [Google Scholar]
- Hamada R, Yoshida Y, Kuwano A, Mishima I, Igata A. Auditory brainstem responses in fetal organic mercury poisoning (in Japanese) Shinkei-Naika. 1982;16:282–285. [Google Scholar]
- Harada M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harper CR, Jacobson TA. Usefulness of omega-3 fatty acids and the prevention of coronary heart disease. Am J Cardiol. 2005;96:1521–1529. doi: 10.1016/j.amjcard.2005.07.071. [DOI] [PubMed] [Google Scholar]
- Hearn TL, Sgoutas SA, Hearn JA, Sgoutas DS. Polyunsaturated fatty acids and fat in fish flesh for selecting species for health benefits. J Food Sci. 1987;52:1209–11. [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- Hightower J, Moore D. Mercury levels in high-end consumers of fish. Environ Health Perspect. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat M, Nolde N, Fajon V, Jerb V, Logar M, Lojen S, Jacimovic R, Falnoga I, Qu L, faganeli J, Drobne D. Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Tot Environ. 2003;304:231–256. doi: 10.1016/S0048-9697(02)00572-7. [DOI] [PubMed] [Google Scholar]
- Hunter D, Russell DS. Focal celebral and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurol Neurosurg Psychiatr. 1954;17:235–41. doi: 10.1136/jnnp.17.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igata A. Epidemiological and Clinical Features of Minamata Disease. Environ Res. 1993;63:157–69. doi: 10.1006/enrs.1993.1137. [DOI] [PubMed] [Google Scholar]
- Innis SM. Essential fatty acids in growth and development. Prog Lip Res. 1991;30:39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- Innis SM, Palaty J, Vaghri Z, Lockitch G. Increased levels of mercury associated with high fish intakes among children from Vancouver, Canada. J Pediatr. 2006;148:759–763. doi: 10.1016/j.jpeds.2006.02.001. [DOI] [PubMed] [Google Scholar]
- International Council for the Control of Iodine Deficiency Disorder (ICCIDD). Iodine Deficiency Disorder (IDD) [Accessed on 4/10/2007]; http://www.indorgs.virginia.edu/iccidd (updated August 18, 2005)
- IOM/NAS (Institute of Medicine/National Academy of Sciences) Food and Nutrition Board. Washington, DC: The National Academies Press; 2007. Seafood choices: balancing benefits and risks. [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, Dowler JK. The transfer of polycholorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. J Public Health. 1984;74:378–9. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Concato J, Leventhal JM. How good is the evidence linking breastfeeding and intelligence? Pediatr. 2002;109:1044–1053. doi: 10.1542/peds.109.6.1044. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, Lisowska-Miszczyk I, Szpanowska-Wohn A, Rauh V, Skolicki Z, Kaim I, Perera F. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16:439–447. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- Kalra V, Grover J, Ahuja GK, Rathi S, Khurana DS. Vitamin E deficiency and associated neurological deficits in children with protein-energy malnutrition. J Trop Pediatr. 1998;44:291–295. doi: 10.1093/tropej/44.5.291. [DOI] [PubMed] [Google Scholar]
- Keiding N, Budtz-Jørgensen E, Grandjean P. Prenatal methylmercury exposure in the Seychelles [letter] Lancet. 2003;362:664–665. doi: 10.1016/S0140-6736(03)14166-9. [DOI] [PubMed] [Google Scholar]
- Kjellström T, Kennedy P, Wallis S, Mantell C. State 1: Preliminary tests at age 4. (Report 3080) Stockholm: National Swedish Environmental Protection Board; 1986. Physical and mental development of children with prenatal exposure to mercury from fish. [Google Scholar]
- Kjellström T, Kennedy P, Wallis S. Stage 2, interviews and psychological tests at age 6. (Report 3642) Stockholm: National Swedish Environmental Protection Board; 1989. Physical and mental development of children with prenatal exposure to mercury from fish. [Google Scholar]
- Kjellström T. Methylmercury exposure and intellectual development in vulnerable groups on New Zealand; Proceedings of the US-Japan workshop; Nov. 2000; Minamata, Japan, National Institute for Minamata Disease. 2000. [Google Scholar]
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, Igumnov S, Fombonne E, Bogdanovich N, Ducruet T, Collet JP, Chalmers B, Hodnett E, Davidovsky S, Skugarevsky O, Trofimovich O, Kozlova L, Shapiro S Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. Breastfeeding and child cognitive development. Arch Gen Psychiatry. 2008;65:578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Bosschieter FB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312:1205–9. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- Lanting CI, Weisglas-Kuperus N, Touwen BCL, Boersma ER. Breastfeeding and neurological outcome at 42 months. Acta Paediatr. 1998;87:1224–9. doi: 10.1080/080352598750030870. [DOI] [PubMed] [Google Scholar]
- Levenson CW, Axelrad DM. Too much of a good thing? Update on fish consumption and mercury exposure. Nutr Rev. 2006;64:139–145. doi: 10.1111/j.1753-4887.2006.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, Jimenez R, Mora LA, Gomes I, Krauskoph D. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatr. 1987;79:981–995. [PubMed] [Google Scholar]
- Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C. Breast milk and subsequent intelligence quotient in children born preterm. Lancet. 1992;339:261–4. doi: 10.1016/0140-6736(92)91329-7. [DOI] [PubMed] [Google Scholar]
- Ma J, Stampfer MJ. Body iron stores and coronary heart disease. Clin Chem. 2002;48:601–3. [PubMed] [Google Scholar]
- Maberly GF. Idoine deficiency disorders: contemporary scientific issues. J Nutr. 1994a;124:1473S–1478S. doi: 10.1093/jn/124.suppl_8.1473S. [DOI] [PubMed] [Google Scholar]
- Maberly FG, Trowbridge FL, Yip R, Sullivan KM, West CE. Programs against micronutrient malnutrition: ending hidden hunger. Annu Rev Public Health. 1994b;15:277–301. doi: 10.1146/annurev.pu.15.050194.001425. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004a;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR. Fish and shellfish as dietary sources of methylmercury and the -3 fatty acids, eicosahexaenoic acid and docosahexaenoic acid: risks and benefits. Env Res. 2004b;95:414–428. doi: 10.1016/j.envres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mahaffey KR, Schoeny R. Maternal seafood consumption and children’s development. Lancet. 2007;370:216–217. doi: 10.1016/S0140-6736(07)61116-7. [DOI] [PubMed] [Google Scholar]
- Marsh DO, Turner MD, Smith JC, Perez VMH, Allen P, Richdale N. Fetal MeHg study in a Peruvian fish eating population. Neurotoxicity. 1995;16:717–726. [PubMed] [Google Scholar]
- Martinez M, Ballabriga A, Gil-Gibernou JJ. Lipids of the developing human retina: I. Total fatty acids, plasmalogens, and fatty acid composition of ethanolamine and choline phosphoglycerides. J Neurosci Res. 1988;20:484–490. doi: 10.1002/jnr.490200412. [DOI] [PubMed] [Google Scholar]
- Martinez M. Developmental profiles of polyunsaturated fatty acids in the brain of normal infants and patients with peroxisomal diseases: severe deficiency of docosahexaenoic acid in Zellweger’s and Pseudo-Zellweger’s syndromes. In: Simopoulos AP, Kifer RR, Martin RE, Barlow SM, editors. Health effects of omega-3 polyunsaturated fatty acids in seafoods, world review of nutrition and dietetics. Vol. 66. Basel, Switzerland: Karger Publishing Press; 1991. pp. 87–102. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Ruiz de Oña C, Obregón MJ, Escobar del Rey F. Models of fetal iodine deficiency. In: Delong GR, Robbins J, Condliffe PG, editors. Iodine and the brain. New Yor: Plenum Press; 1989. pp. 187–201. [Google Scholar]
- Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly É, Jacobson SW, Jacobson JL. Prenatal exposure of the Northern Quebec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109:1291–1299. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K, Weihe P, Araki S, Budtz-Jørgensen E, Grandjean P. Evoked Potentials in Faroese children prenatally exposed to methylmercury. Neurotoxicol Teratol. 1999a;21:471–472. doi: 10.1016/s0892-0362(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Renzoni A, Debes F, Vasconcelos R, Zino F, Araki S, Jørgensen PJ, White RF, Grandjean P. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotoxicol Teratol. 1999b;21:343–348. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Grandjean P. Delayed brainstem auditory evoked potential latencies in 14-year-old children exposed to methylmercury. J Pediatr. 2004;144:177–183. doi: 10.1016/j.jpeds.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Marsh DO, Cernichiari E, Lapham LW, Berlin M, Clarkson TW. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicol. 1995;16:711–716. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J Nutr. 2007;137:2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- NIEHS. Workshop organized by the Committee on Environmental and Natural Resources (CENR), Office of Science and Technology Policy (OSTP), The White House: Scientific Issues Relevant to Assessment of Health Effects from Exposure to Methylmercury, November 18–20, 1998. [Accessed 4/16/07]; Available from: http://ntp.niehs.nih.gov/index.cfm?objectid=03614B65-BC68-D2314E915F93AF9A6872#execsumm.
- Nilsen DW, Albrekten G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate o fn-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74:50–56. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- Nordoy A, Dyerberg J. n-3 fatty acids in health and disease. J Inter med. 1989;225(suppl 1):S1–3. doi: 10.1111/j.1365-2796.1989.tb01428.x. [DOI] [PubMed] [Google Scholar]
- NRC. Toxicological effects of methylmercury. Washington: National Research Council; 2000. [Google Scholar]
- O’Brien JS, Fillerup DL, Mean JF. Quantification of fatty acid and fatty aldehyde composition of ethanolamine, choline and serine phosphoglycerides in human cerebral gray and white matter. J Lipid Res. 1964;3:329–330. [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113:376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena C, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and chid cognition at age 3 years in a US cohort. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- Pollitt E. Iron deficiency and cognitive function. Annu Rev Nutr. 1993;13:521–537. doi: 10.1146/annurev.nu.13.070193.002513. [DOI] [PubMed] [Google Scholar]
- Raper NR, Cronin FJ, Exler J. Omega-3 fatty acid content of the US food supply. J Am Coll Nutr. 1992;11:304–8. doi: 10.1080/07315724.1992.10718231. [DOI] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–41. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008 doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaeonoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–9. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC. Breast-feeding and cognitive development. Early Hum Develop. 1993;31:181–193. doi: 10.1016/0378-3782(93)90194-y. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. 2. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- Sacks FM, Stone PH, Gibson CM, Siverman DI, Rosner B, Pasternak RC. Controlled trial of fish oil for regression of human coronary atherosclerosis. HARP Research Group. J Am Coll Cardiol. 1995;25:1492–1498. doi: 10.1016/0735-1097(95)00095-l. [DOI] [PubMed] [Google Scholar]
- Saldeen TG, Mehta JL. Dietary modulations in the prevention of coronary artery disease: a special emphasis on vitamins and fish oil. Curr Opin Caridol. 2002;17:559–567. doi: 10.1097/00001573-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Seppänen K, Kantola M, Suntioinen S, Korpela H. Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. Br Med J. 1991;302:756–760. doi: 10.1136/bmj.302.6779.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86:803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid, peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any deaths in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Salonen JT, Seppänen K, Lakka TA, Salonen R, Kaplan GA. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis. 2000;148:265–273. doi: 10.1016/s0021-9150(99)00272-5. [DOI] [PubMed] [Google Scholar]
- Schaller JL. Mercury and fish oil supplements. MedGenMed. 2001;3:20. [PubMed] [Google Scholar]
- Sempos CT, Looker AC, Gillum RF, Makuc DM. Body iron stores and the riks of coronary heart disease. N Engl J Med. 1994;330:1119–1124. doi: 10.1056/NEJM199404213301604. [DOI] [PubMed] [Google Scholar]
- Shamlaye CF, Marsh DO, Myers GJ, Cox C, Davidson PW, Choisy O, Cernichiari E, Choi A, Tanner MA, Clarkson TW. The Seychelles child development study on neurodevelopmental outcomes in children following in utero exposure to methylmercury from a maternal fish diet: background and demographics. Neurotoxicol. 1995;16:597–612. [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival – 4. Cardiovasc Drugs Ther. 1997;11:485–491. doi: 10.1023/a:1007757724505. [DOI] [PubMed] [Google Scholar]
- Sokol RJ. Vitamin E and neurologic function in man. Free Radic Biol Med. 1989;6:189–207. doi: 10.1016/0891-5849(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jørgensen P, Bjerve K, Brock J, Heinzow B, Budtz-Jørgensen, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Bonham MP, Davidson PW, Myers GJ, Thurston SW, Clarkson TW, Stokes-Riner A, Janciuras J, Sloane-Reeves J, Cernichiari E, Shamlaye CF, Duffy EM, Robson PJ, Wallace JMW. Long-chain polyunsaturated fatty acids and mercury. Oral presentation at the International Conference on Fetal Programming and Developmental Toxicity; Faroe Islands. 20–24 May, 2007; 2007. [Accessed 5/20/07]. Abstract available: http://www.pptox.dk/portals/0/o39.pdf. [Google Scholar]
- Sweeney M, Peck K, Schantz L, Gardiner C, Gasior M, Batizy H, Fitzpatrick D, Kostyniak J. Contaminant profiles in southeast Asian immigrants consuming fish from Northeastern Wisconsin waters. Epidemiol. 2006;17(Suppl):S340. doi: 10.1016/j.envres.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson BG, Schütz A, Nilsson A, Åkesson I, Åkesson B, Skerfving S. Fish as a source of exposure to mercury and selenium. Sci Total Environ. 1992;126:61–74. doi: 10.1016/0048-9697(92)90484-a. [DOI] [PubMed] [Google Scholar]
- Tinoco J, Miljanich P, Medwadowski B. Depletion of docosahexaenoic acid in retinal lipids of rats fed a linolenic acid-deficient, linoleic acid-containing diet. Biochim Biophys Acta. 1977;486:575–578. doi: 10.1016/0005-2760(77)90111-4. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) [accessed 2 March, 2007];National listing of fish advisories. 2004 Available: http://www.epa.gov/waterscience/fish/advisories/fs2004.pdf.
- Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Volkonen VP, Seppänen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- Visioli F, Rise P, Barassi MC, Marangoin F, Galli C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids. 2003;38:415–418. doi: 10.1007/s11745-003-1077-x. [DOI] [PubMed] [Google Scholar]
- von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary ω-3 fatty acids on coronary atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- Walkowiak J, Wiener JA, Fastabend A, Heinzow B, Krämer U, Schmidt E, Steingrüber HJ, Wundram S, Winneke G. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet. 2001;358:1602–1607. doi: 10.1016/S0140-6736(01)06654-5. [DOI] [PubMed] [Google Scholar]
- Wen ZY, Chen F. Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnol Adv. 2003;21:273–294. doi: 10.1016/s0734-9750(03)00051-x. [DOI] [PubMed] [Google Scholar]
- Whanger PD. Selenium in the treatment of heavy metal poisoning and chemical carcinogens. J Trace Elem Electrolytes Health Dis. 1992;6:209–21. [PubMed] [Google Scholar]
- Whanger PD. Selenium and the brain: a review. Nutr Neurosci. 2001;4:81–97. doi: 10.1080/1028415x.2001.11747353. [DOI] [PubMed] [Google Scholar]
- Willatts P, Forsyth JS. The role of long-chain polyunsaturated fatty acids in infant cognitive development. Prostaglandins Leukot Essent Fatty Acids. 2000;63:95–100. doi: 10.1054/plef.2000.0198. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Selenium; Geneva. Switzerland: 1987. [Google Scholar]
- Yamamoto R, Suzuki T. Effects of artificial hair-waving on hair mercury values. Int Arch Occup Environ Health. 1978;42:1–9. doi: 10.1007/BF00385706. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Matsumoto M, Yamaguchi M, Hachiya N. Current hair mercury levels in the Japanese: survey in five districts. Tohoku J Exp Med. 2003;199:161–169. doi: 10.1620/tjem.199.161. [DOI] [PubMed] [Google Scholar]
- Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh CC, Spiegelman D, Stampfer MJ, Willett WC. Mercury and the risk of coronary heart disease in men. N Engl J Med. 2002;347:1755–60. doi: 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]
- Zhou SJ, Baghurst P, Gibson RA, Makrides M. Home environment, not duration of breast-feeding, predicts intelligence quotient of children at four years. Nutrition. 2007;23:236–241. doi: 10.1016/j.nut.2006.12.011. [DOI] [PubMed] [Google Scholar]


