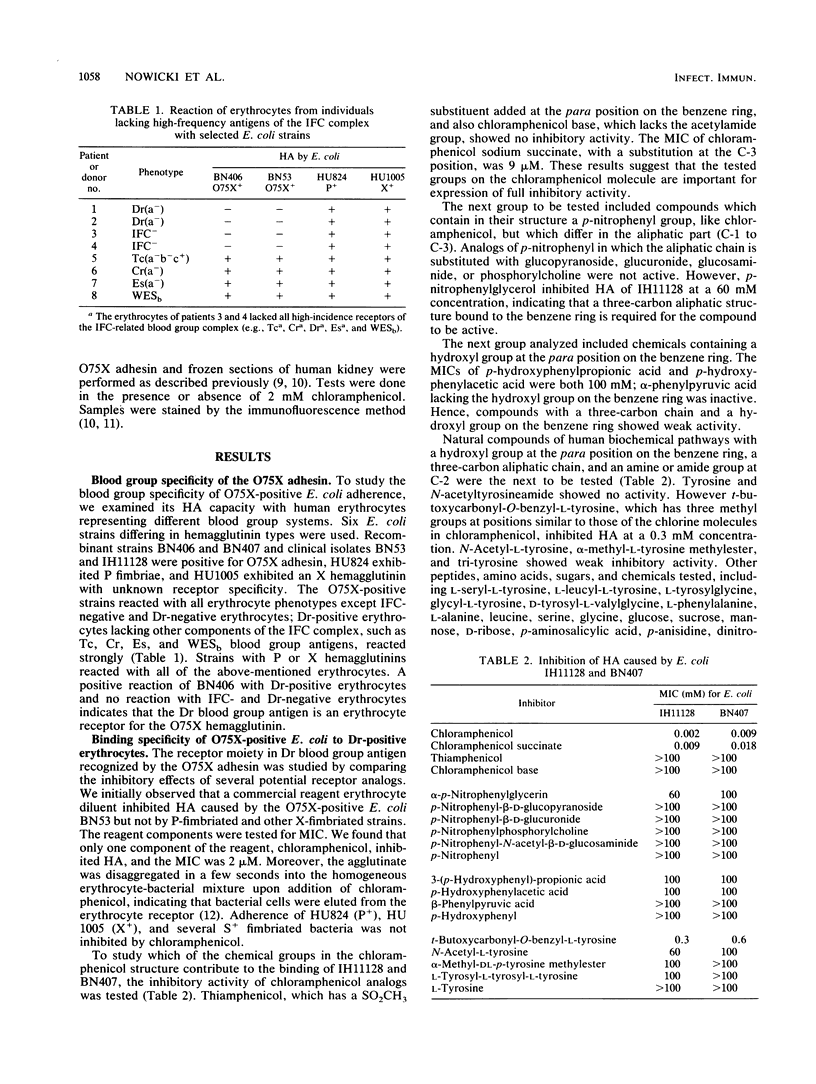
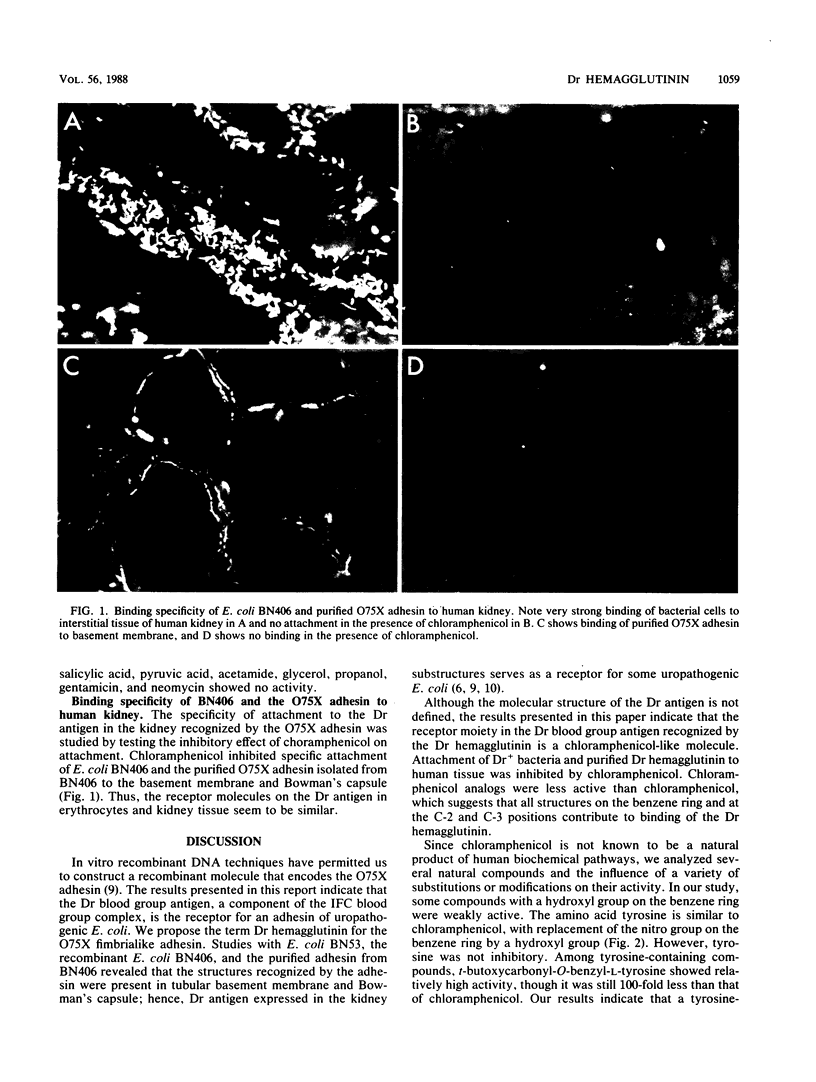
Abstract
A receptor moiety and blood group substance recognized by the O75X adhesin was studied. Well-defined erythrocytes representing different blood group systems and bacterial derivatives carrying plasmid pBJN406 encoding the adhesin were used in a direct hemagglutination assay. We showed that Dr blood group antigen, a component of the IFC blood group complex, is the receptor for the O75X fimbrialike adhesin (Dr hemagglutinin) of uropathogenic Escherichia coli. The molecule recognized by the Dr hemagglutinin on Dr blood group substance is a chloramphenicol-like structure. The inhibitory effect of the active compounds indicates that a tyrosine-containing molecule could be a natural receptor for the Dr hemagglutinin. Dr blood group substance was found in tubular basement membrane and Bowman's capsule of the human kidney. Specific attachment of a Dr hemagglutinin-positive bacterial strain to the kidney substructures was inhibited by chloramphenicol.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daniels G. L., Tohyama H., Uchikawa M. A possible null phenotype in the Cromer blood group complex. Transfusion. 1982 Sep-Oct;22(5):362–363. doi: 10.1046/j.1537-2995.1982.22583017458.x. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Levene C., Harel N., Lavie G., Greenberg S., Laird-Fryer B., Daniels G. L. A "new" phenotype confirming a relationship between Cra and Tca. Transfusion. 1984 Jan-Feb;24(1):13–15. doi: 10.1046/j.1537-2995.1984.24184122551.x. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Barrish J. P., Korhonen T., Hull R. A., Hull S. I. Molecular cloning of the Escherichia coli O75X adhesin. Infect Immun. 1987 Dec;55(12):3168–3173. doi: 10.1128/iai.55.12.3168-3173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Holthöfer H., Saraneva T., Rhen M., Väisänen-Rhen V., Korhonen T. K. Location of adhesion sites for P-fimbriated and for 075X-positive Escherichia coli in the human kidney. Microb Pathog. 1986 Apr;1(2):169–180. doi: 10.1016/0882-4010(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Rhen M., Väisänen-Rhen V., Pere A., Korhonen T. K. Immunofluorescence study of fimbrial phase variation in Escherichia coli KS71. J Bacteriol. 1984 Nov;160(2):691–695. doi: 10.1128/jb.160.2.691-695.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Burrows M. R., Sellwood R., Gibbons R. A. A genetic basis for resistance to enteric disease caused by E. coli. Nature. 1975 Sep 11;257(5522):135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hull R., Falkow S., Leffler H. Target cell specificity of wild-type E. coli and mutants and clones with genetically defined adhesins. Prog Food Nutr Sci. 1983;7(3-4):75–89. [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]



