Abstract
Ca2+ release-activated Ca2+ (CRAC) channels underlie sustained Ca2+ signaling in lymphocytes and numerous other cells following Ca2+ liberation from the endoplasmic reticulum (ER). RNAi screening approaches identified two proteins, Stim1, 2 and Orai3-5, that together form the molecular basis for CRAC channel activity6, 7. Stim senses depletion of the ER Ca2+ store and physically relays this information by translocating from the ER to junctions adjacent to the plasma membrane (PM)1, 8, 9, and Orai embodies the pore of the PM calcium channel10-12. A close interaction between Stim and Orai, identified by co-immunoprecipitation12 and by Förster resonance energy transfer13, is involved in opening the Ca2+ channel formed by Orai subunits. Most ion channels are multimers of poreforming subunits surrounding a central channel, which are preassembled in the ER and transported in their final stoichiometry to the PM. Here we show by biochemical analysis after cross-linking in cell lysates and in intact cells, and by non-denaturing gel electrophoresis without cross-linking that Orai is predominantly a dimer in the PM under resting conditions. Moreover, single-molecule imaging of GFP-tagged Orai expressed in Xenopus oocytes revealed predominantly two-step photo-bleaching, consistent again with a dimeric basal state. In contrast, co-expression of GFP-tagged Orai with the C-terminus of Stim as a cytosolic protein to activate the Orai channel without inducing Ca2+ store depletion or clustering of Orai into punctae yielded predominantly four-step photobleaching, consistent with a tetrameric stoichiometry of the active Orai channel. Interaction with the C-terminus of Stim thus induces Orai dimers to dimerize, forming a tetramer that constitutes the Ca2+-selective pore. This represents a novel mechanism in which assembly and activation of the functional ion channel are mediated by the same triggering molecule.
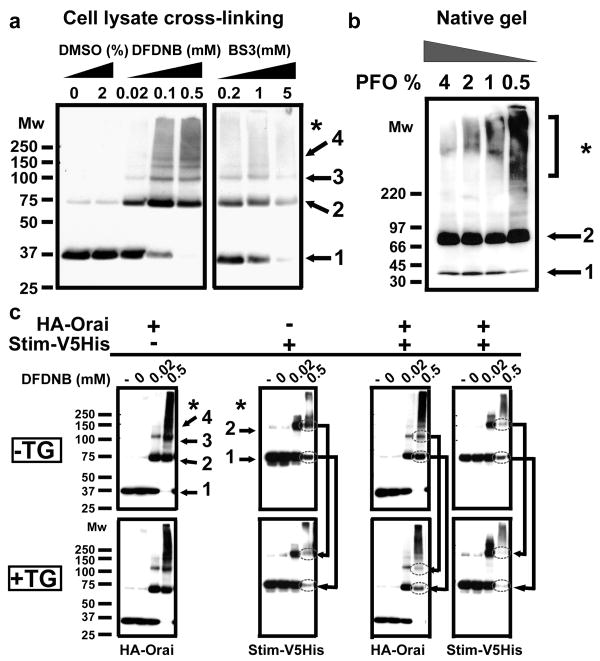
As an atypical four transmembrane-spanning protein with no sequence similarity to any other ion channel, Orai's subunit organization and mode of activation remain undefined. Knowledge of the stoichiometry of Orai is crucial to understand the mechanisms of channel assembly, gating and ion permeation, but previous studies led to differing conclusions, with biochemical experiments suggesting that the Orai complex is a dimer in both resting and thapsigargin-treated cells14, whereas functional measurements of expressed tandem Orai multimers implicate a tetramer as the active CRAC channel pore15. To resolve this issue we began by applying biochemical techniques used in the past to solve the pore stoichiometry of other channels16-19: co-immunoprecipitation, chemical cross-linking of intact and lysed cells, and polyacrylamide gel electrophoresis (PAGE) under non-dissociative conditions. Reciprocal co-immunoprecipitation confirmed that Orai subunits bearing different tags can co-assemble11, 14 (Supplementary Fig. 1). Following treatment of total cell lysates of Drosophila S2 cells transfected with HA-tagged Orai (Orai), increasing concentrations of three different lysine-reactive homobifunctional reagents revealed cross-linked species on SDS-PAGE of ≈80, ≈120, and ≈160 kDa; consistent with molecular masses of Orai dimers, trimers and tetramers, respectively (Fig. 1a). The band corresponding to Orai dimers was always the most intense. This pattern was seen also in intact Orai-transfected S2 cells using both homobifunctional cysteine- or lysine-reactive cross-linkers, again suggesting that Orai dimers are the main form present in living cells (Supplementary Fig. 2a). The relative mobility of each band decreased as a logarithmic function of the estimated number of cross-linked subunits, indicating that the cross-linked products are integral homomultimers of the monomeric subunit18 (Supplementary Fig. 2b). This was confirmed using a functional GFP-Flag tag Orai construct; the GFP tag increased the apparent molecular mass of the Orai monomer by the predicted amount of ∼26 kDa (Supplementary Fig. 2c, d). If each oligomeric species were purely composed of Orai, their sizes should be directly proportional to an integer multiple of the ≈68 kDa GFP-Orai monomer, exactly as observed. The absence of graded formation of oligomers with a higher order than dimers as a function of increasing cross-linker concentration (Fig. 1a; Supplementary Fig. 2a) or time of cross-linker incubation (Supplementary Fig. 3) suggests that the Orai oligomeric state is a dimer in resting S2 cells. The predominant homodimeric stoichiometry was further confirmed in S2 and HEK-293 cells by perfluorooctanoic (PFO) native gel electrophoresis. PFO is a mild non-denaturing and non-dissociative detergent for molecular complexes that has been successfully used to determine the quaternary structure of various membrane proteins19. Under mild solubilization conditions Orai was almost exclusively observed in a dimeric state (Fig. 1b). Varying the PFO to protein and/or lipid ratio, the time or the temperature of solubilization in PFO did not lead to the appearance of higher order Orai oligomers (Supplementary Fig. 4).
Figure 1. Orai is present mainly as homodimers in resting S2 cells.
Each panel is representative of at least 3 independent experiments. Numbers represent assigned state of oligomerization: 1, monomer; 2, dimer; 3, trimer; 4, tetramer; * high-order aggregates. a, Determination of Orai oligomeric structure using chemical cross-linking. DFDNB (1,5-difluoro-2,4-dinitrobenzene, membrane-permeant) and BS3 (bis(sulfosuccimidyl)suberate, membrane-impermeant) were incubated with HA-Orai transfected S2 cells lysates and the sizes of the cross-linked products were analyzed by SDS-PAGE on 4-12% gradient gels. Orai oligomers, from dimer to tetramer, were observed with dimer always being the predominant population. Similar results were obtained in intact cells using DSP (dithiobis succinimidylpropionate, membrane-permeant, data not shown), DFDNB (see Supplementary Fig. 2, 3), and a cysteine-reactive cross-linker BMH (maleimide 1,6-bismaleimidohexane, membrane permeable). b, Confirmation of Orai dimerization using PFO-PAGE. HA-Orai transfected S2 cell lysates were incubated with sample buffer containing different PFO concentration for 30 minutes at room temperature before electrophoresis. c, DFDNB cross-linking of total cell lysates of S2 cells tranfected with HA-Orai only, Stim-V5His only or co-transfected. Labels indicate untreated cells (-), DMSO vehicle control (0) and concentrations of DFDNB. Arrows show the reduction in Stim and Orai low-order oligomers upon Ca2+ store depletion by TG (1.5 μM TG for 15 min).
When Stim and Orai are co-expressed in Drosophila S2 cells, a greatly amplified CRAC current is recorded after store depletion4. In order to analyze Orai stoichiometry when the CRAC channel is functional, chemical cross-linking experiments were performed on S2 cells transfected with Stim and Orai, using total cell lysates of resting cells and cells treated with thapsigargin (TG) to deplete the Ca2+ stores (Fig. 1c). When Orai was transfected alone no obvious change in the cross-linking pattern was observed with or without TG treatment. In contrast, store depletion caused a decrease of the cross-linked dimer intensity of a V5His-tagged Stim protein (Stim) expressed alone, and a decrease of intensity of both Orai and Stim dimers in cotransfected cells Since a constant amount of protein was loaded in each lane, most of the Stim protein, and the Orai protein when co-expressed with Stim, were likely present as very high molecular weight cross-linked aggregates that did not enter the gel.
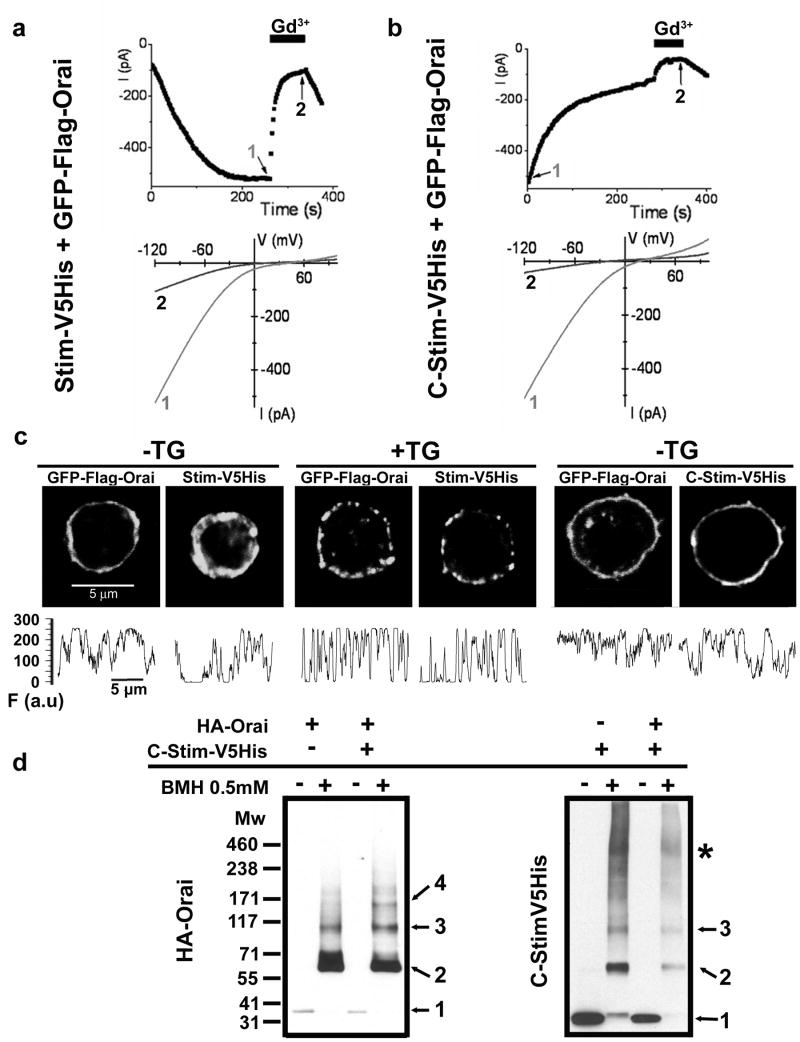
Because aggregation of Stim and Orai into macromolecular complexes precluded the determination of Orai stoichiometry in the active state, we sought a way to activate the CRAC channel without inducing higher order Orai cluster formation and, in addition, to test whether aggregation of Orai into punctae is a requirement for CRAC channel function. The C-terminal portion of STIM1, expressed as a cytosolic protein, activates CRAC current constitutively in Jurkat T cells20 and in HEK cells co-transfected with Orai113, 21. Expression of Drosophila C-terminal Stim bearing a V5-His tag (C-Stim) induced constitutive Ca2+ influx in S2 cells (Supplementary Fig. 5). We compared currents in S2 cells co-transfected with GFP-Orai and either full-length Stim or C-Stim. With co-transfected Stim, GFP-Orai produced amplified CRAC currents that developed upon passive store depletion (Fig. 2a), similar in time course (half-time of 83±24 sec, n = 11 cells) but approximately ten-fold larger in amplitude than native CRAC currents22 and consistent with results described previously for Stim + untagged Orai4. In contrast, cells transfected with C-Stim + GFP-Orai exhibited a robust pre-activated CRAC-like current immediately upon breaking in to initiate whole-cell recording (Fig. 2b, Supplementary Fig. 6a). The pre-activated current was identical to native or amplified CRAC currents in its inwardly rectifying I-V shape, selectivity for Ca2+, and sensitivity to block by 5 nM Gd3+ (Fig. 2a, b; Supplementary Fig. 6b, c). However, the pre-activated current induced by C-Stim + GFP-Orai declined to a half-maximal value in 34±9 sec (n = 14 cells), likely due to pipette dialysis resulting in dilution and unbinding of cytosolic C-Stim, which, unlike full length Stim, is not constrained to the ER membrane while interacting with Orai. Indeed, when small pipettes with higher series resistance and correspondingly slower diffusional access were employed, the decline of pre-activated current proceeded more slowly (Supplementary Fig. 6d, e).
Figure 2. The C-terminus of Stim (C-Stim) constitutively activates Orai without forming punctae.
a, b Time course (upper) and I-V curves (lower) comparing CRAC current in representative cells co-transfected with Stim + GFP-Orai (a) or with C-Stim + GFP-Orai (b). Application of 5 nM Gd3+ reversibly blocked most of the current in cells transfected with either Stim + GFP-Orai or C-Stim + GFP-Orai. c, Subcellular localization of GFP-Orai together with Stim-V5His or C-Stim-V5His in resting (StimV5His and C-StimV5His) or store-depleted (2 μM TG for 15 min, Stim-V5His only) co-transfected S2 cells. Puncta formation was only observed upon store-depletion in cells expressing StimV5His and GFP-Orai. The panels underneath each picture display fluorescence intensity profiles (F) for Orai and Stim obtained from the same regions of interest, tracing the perimeter of each cell clockwise from top. To quantify the extent to which GFP-Orai was inhomogeneously distributed we calculated the ratio of fluorescence variance/mean fluorescence from profiles like those illustrated. Mean ratio values for GFP-Orai (±SEM; n=6 cells for each condition) were: StimV5His, -TG, 29.03±2.9; StimV5His, +TG, 62.9±4.5 (p = 0.00002); C-Stim-V5His, -TG, 22.4±3.7 (not significantly different from StimV5His). d, BMH cross-linking in intact S2 cells transfected with HA-Orai only, C-Stim-V5His only or co-transfected. Numbers represent inferred state of oligomerization: 1, monomer; 2, dimer; 3, trimer; 4, tetramer; *, higher order aggregates. The cross-linking pattern of Orai showed a clear tetrameric population when co-expressed with C-Stim, but not in the absence of C-Stim. The cross-linking profile of C-Stim was not affected by the presence of Orai, and revealed a majority of dimers and trimers.
We next examined the localization of GFP-Orai in relation to co-expressed C-Stim or Stim (Fig. 2c, Supplementary Fig. 7). Immunostaining of cytosolic C-Stim showed it to be present in a ring near the surface membrane, co-localized with GFP-Orai and suggestive of a constitutive coupling. Importantly, no punctae of C-Stim and co-expressed GFP-Orai were observed, even though CRAC channels were constitutively active. In contrast to this homogeneous distribution of C-Stim and co-expressed Orai, co-localized Stim and Orai showed distinct punctae as expected following Ca2+-store depletion. The C-terminal portion of Stim includes the coiled-coil motif involved in dimerization but lacks the N-terminal SAM domain responsible for higher order Stim aggregation after store depletion. We have previously shown that store depletion induces a dynamic coupling between Stim and Orai by co-immunoprecipitation12. Here, we further demonstrate that Drosophila C-Stim physically interacts with Orai independent of the ER calcium store content (Supplementary Fig. 8). Moreover, Western blots following BMH cross-linking of intact cells revealed that C-Stim induces a shift toward higher order Orai homomultimers, including a clear tetramer population that was not observed when Orai was expressed alone (Fig. 2d). Collectively, these experiments show that C-Stim expressed as a cytoplasmic protein associates with and constitutively activates Orai subunits in the PM without forming punctae, and provide evidence that the oligomeric state of Orai depends upon C-Stim binding.
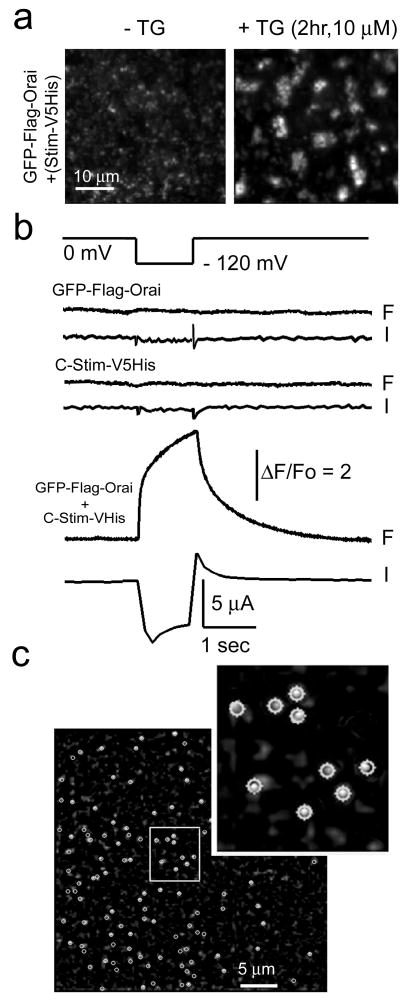
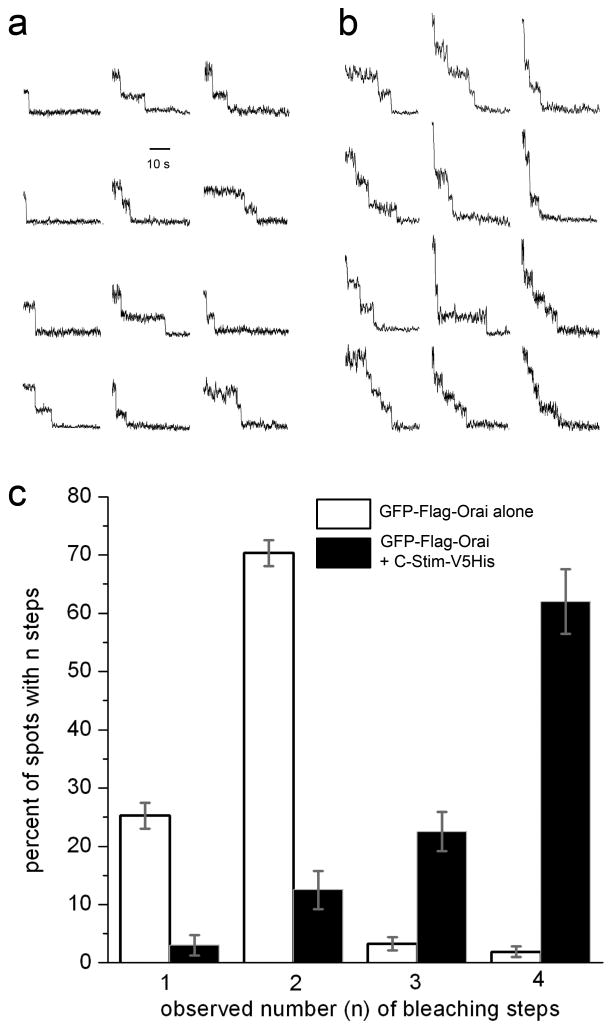
To determine Orai stoichiometry at the single-molecule level in the native membrane environment of intact cells we used a recently developed method23, employing total internal reflection microscopy (TIRFM) to image bleaching steps of individual GFP-tagged Orai. Xenopus oocytes were injected with cRNA for GFP-Orai with or without coincident injection of cRNA for Stim or C-Stim. When Stim was co-expressed, depletion of the Ca2+ store with TG resulted in clustering of GFP-Orai in punctae (Fig 3a), making single molecules difficult to resolve. However, consistent with the above results in Drosophila S2 cells, oocytes expressing both GFP-Orai and C-Stim showed large Ca2+ influx as assessed by Ca2+ fluorimetry, together with activation of endogenous Ca2+-dependent Cl- current; whereas neither Orai nor C-Stim alone were effective (Fig. 3b). Moreover, TIRFM imaging of GFP-Orai-expressing oocytes revealed numerous, diffraction-limited fluorescent spots (Fig. 3c), that were absent in non-injected oocytes and increased in density with time of expression. Continuous exposure to laser excitation resulted in stepwise decrements of fluorescence at these spots (Supplementary Movies), corresponding to bleaching of individual GFP molecules23. Different spots showed varying numbers of bleaching steps, ranging from one to a maximum of four (Fig. 4a,b). Notably, estimates of the mean number of GFP molecules per spot made in this way differed markedly depending on expression of C-Stim. In oocytes expressing GFP-Orai alone, a majority (∼70%) of spots showed two steps to complete bleaching (Fig. 4a,c), consistent with biochemical observations in S2 cells, whereas with co-expression of C-Stim most spots (∼62%) showed four-step bleaching (Fig. 4b,c). The small proportions of spots that showed one- or three-step bleaching may reflect instances of near-simultaneous stochastic bleaching steps that could not separately resolved or expression of non-fluorescent GFP molecules23. The optical resolution of the microscope (ca. 250 nm) is inadequate to determine whether a spot showing 4 bleaching steps is truly a tetramer or, for example, two distinct dimers linked by C-Stim. We favor the former interpretation based on evidence15 that expression of an Orai1 tandem tetramer construct forms functional CRAC channels, and that CRAC is inhibited when one subunit in the tetramer is replaced by a dominant-negative Orai. We thus conclude that Orai is present in the membrane predominantly as dimers under basal conditions, and that activation by C-Stim induces association to form tetramers.
Figure 3. Single-molecule photo-bleaching of GFP-Orai in intact oocytes.
a, Store depletion induces formation of Orai punctae in Xenopus oocytes. Images were obtained by TIRF microscopy of oocytes expressing GFP-Orai together with StimV5His and show 40 × 40 μm regions in the animal hemisphere before (left) and 2 hr after (right) bath application of 10 μM TG in zero-calcium Ringer's solution. b, Oocytes expressing GFP-Orai together with C-Stim showed strong Ca2+ influx, whereas this was absent with expression of GFP-Orai or C-Stim alone. Pairs of traces show cytosolic [Ca2+] as reported by normalized fluorescence pseudoratio changes of Fluo-4 (F) and whole-cell voltage-clamp measurements of Ca2+-activated Cl- current (I) in response to a hyperpolarizing step from 0 mV to -120 mV (top trace). c, Representative TIRFM image, acquired before photobleaching, showing fluorescent spots (circled) sparsely distributed in the membrane of an oocyte expressing GFP-Orai together with C-Stim. Inset shows a magnified view of a small region (white box), with circular regions of interest used to measure bleaching steps overlaid on the image.
Figure 4. GFP-Orai forms dimers in the basal state and predominantly tetramers when co-expressed with C-Stim.
a, b Representative examples of single-molecule bleaching records obtained from oocytes expressing, respectively, GFP-Orai alone and GFP-Orai together with C-Stim. c, Histogram shows percentages of spots that showed 1, 2, 3, and 4 bleaching steps in oocytes expressing GFP-Orai alone (open bars) and GFP-Orai plus C-Stim (filled bars). Errors bars indicate ± 1 SEM. Data for GFP-Orai were obtained from 400 spots, 11 imaging records, 6 oocytes; and data for GFP-Orai + C-Stim from 278 spots, 5 imaging records, 3 oocytes. Comparison of bleaching step distributions with and without C-Stim yielded a Chi square value of 590; p < 0.001. This cannot be attributed to an increased likelihood of two GFP-Orai dimers happening to lie indistinguishably close to each other because of increased expression level or C-Stim-induced clustering because fluorescence spots in both conditions showed similar random distributions and densities (respectively, 37±6 and 41±4 spots in a 40×40 μm2 region), and we did not observe spots with >4 bleaching steps as might be expected for a macro-molecular clustering.
Taken together, our results reveal that Orai adopts different quaternary structures depending upon its activation state. In resting cells Orai is present in the PM as dimers, forming stable structural units; but when CRAC is activated Orai is found predominantly as tetramers. This result reconciles biochemical evidence pointing to a stable Orai dimer (reference14 and Fig. 1) with electrophysiological evidence from tandem constructs indicating a tetrameric channel15. Moreover, we show that the coiled-coil C-terminal domain of Stim is sufficient to trigger dimerization of Orai dimers to form the functional tetrameric channel and to activate CRAC influx. However, at present we cannot distinguish whether this dimer to tetramer transition is sufficient to activate Orai channel activity, or if a Stim-induced conformational change in each subunit of Orai is further required for channel activity. The channel assembly and activation mechanism identified here is mechanistically unique in its requirement for an activator protein (Stim) to assemble and to open the Orai tetrameric channel in the PM.
Methods Summary
Drosophila S2 cells (Invitrogen) and HEK293 cells (ATCC) were propagated and transfected (see cDNAs described in Supplementary Information) as described previously2, 12. Chemical cross-linking was performed as described17 with minor modifications (see Supplementary Information). Cross-linking experiments were also performed on living S2 cells directly incubated with different concentrations of cross-linkers. Protein complexes were fractionated by PFO-PAGE as described17, 19 and in Supplementary Information. Co-immunoprecipitations were performed in S2 cells as described12 or on cells solubilized in PBS, 1% NP-40, 5 mM EDTA for Stim-Orai interaction analysis. Equal amounts of protein were immunoprecipitated with the antibodies specified in the figure legends. After extensive washing, eluted samples were analysed by Western blotting. Constructs and tags are described in Supplementary Information.
Transfected S2 cells were selected for whole-cell recording by fluorescence of GFP-Flag-Orai. Handling of C-Stim-transfected cells, solution recipes, voltage stimuli, and data acquisition protocol are included in Supplementary Information.
Single-molecule bleaching experiments were performed on de-folliculated Xenopus oocytes, injected 12-24 hr previously with cRNAs for GFP-Flag-Orai alone or together with C-Stim-V5His. Calcium influx was assayed by applying voltage-clamped hyperpolarizing pulses while monitoring Ca2+-activated Cl- current and fluorescence of intracellularly-loaded fluo-4. Individual GFP-Flag-Orai multimers were visualized by total internal reflection microscopy, and image sequences were analyzed by placing small regions of interest around fluorescence spots to manually count bleaching steps during continuous exposure to 488 nm laser light.
Supplementary Material
accompanies this paper.
Acknowledgments
We thank L. Forrest for assistance in cell culture; T. Holmes and S. Leverrier for useful discussion during the course of the study; A. Amcheslavsky, A. Froger, K. Kalman, and K. Cahalan for help with oocytes; Weihua Jiang for assistance in some of the co-IP experiments; M. Ramjeesingh for help with the PFO-PAGE technique; F-A. Rassendren for the P2X2 construct; and E. Isacoff and M. Ulbrich for discussion and help during a pilot photobleaching study. This work was supported by grants from the National Institutes of Health (M.D.C. and I.P.) and by a fellowship from the George E. Hewitt Foundation (A.P.).
Footnotes
The authors declare no competing financial interests.
Author Contributions A.P. designed and performed cDNA/cRNA constructs, cell biology and all biochemical experiments. A.D. performed all experiments in oocytes and data analysis of single-molecule photobleaching. A.V.Y. was responsible for patch-clamp experiments and analysis. S.L.Z. made the C-Stim construct, performed Ca2+ imaging experiments, and collaborated on co-IP experiments. O.S. provided general technical assistance. I.P. supervised single-molecule experiments on oocytes. M.D.C. provided advice and overall direction, and supervised project planning and execution. A.P., I.P. and M.D.C. wrote the paper.
References
- 1.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahalan MD, et al. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–44. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–7. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 11.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008 doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 14.Gwack Y, et al. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–43. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 15.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–25. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenderop JG, et al. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. Embo J. 2003;22:776–85. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedei N, et al. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–9. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 18.Raab-Graham KF, Vandenberg CA. Tetrameric subunit structure of the native brain inwardly rectifying potassium channel Kir 2.2. J Biol Chem. 1998;273:19699–707. doi: 10.1074/jbc.273.31.19699. [DOI] [PubMed] [Google Scholar]
- 19.Ramjeesingh M, Huan LJ, Garami E, Bear CE. Novel method for evaluation of the oligomeric structure of membrane proteins. Biochem J. 1999;342(Pt 1):119–23. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–10. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SL, et al. Store-dependent and -independent modes regulating CRAC channel activity of human Orai1 and Orai3. J Biol Chem. 2008 doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J Gen Physiol. 2004;123:167–82. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4:319–21. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 25.Newbolt A, et al. Membrane topology of an ATP-gated ion channel (P2X receptor) J Biol Chem. 1998;273:15177–82. doi: 10.1074/jbc.273.24.15177. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen PA, et al. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J Biol Chem. 2002;277:38272–83. doi: 10.1074/jbc.M205348200. [DOI] [PubMed] [Google Scholar]
- 27.Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–43. doi: 10.1016/j.jmb.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 28.Demuro A, Parker I. Optical single-channel recording: imaging Ca2+ flux through individual ion channels with high temporal and spatial resolution. J Biomed Opt. 2005;10:11002. doi: 10.1117/1.1846074. [DOI] [PubMed] [Google Scholar]
- 29.Parker I, Gundersen CB, Miledi R. A transient inward current elicited by hyperpolarization during serotonin activation in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985;223:279–92. doi: 10.1098/rspb.1985.0002. [DOI] [PubMed] [Google Scholar]
- 30.Dargan SL, Demuro A, Parker I. Imaging Ca2+ signals in Xenopus oocytes. Methods Mol Biol. 2006;322:103–19. doi: 10.1007/978-1-59745-000-3_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
accompanies this paper.