Abstract
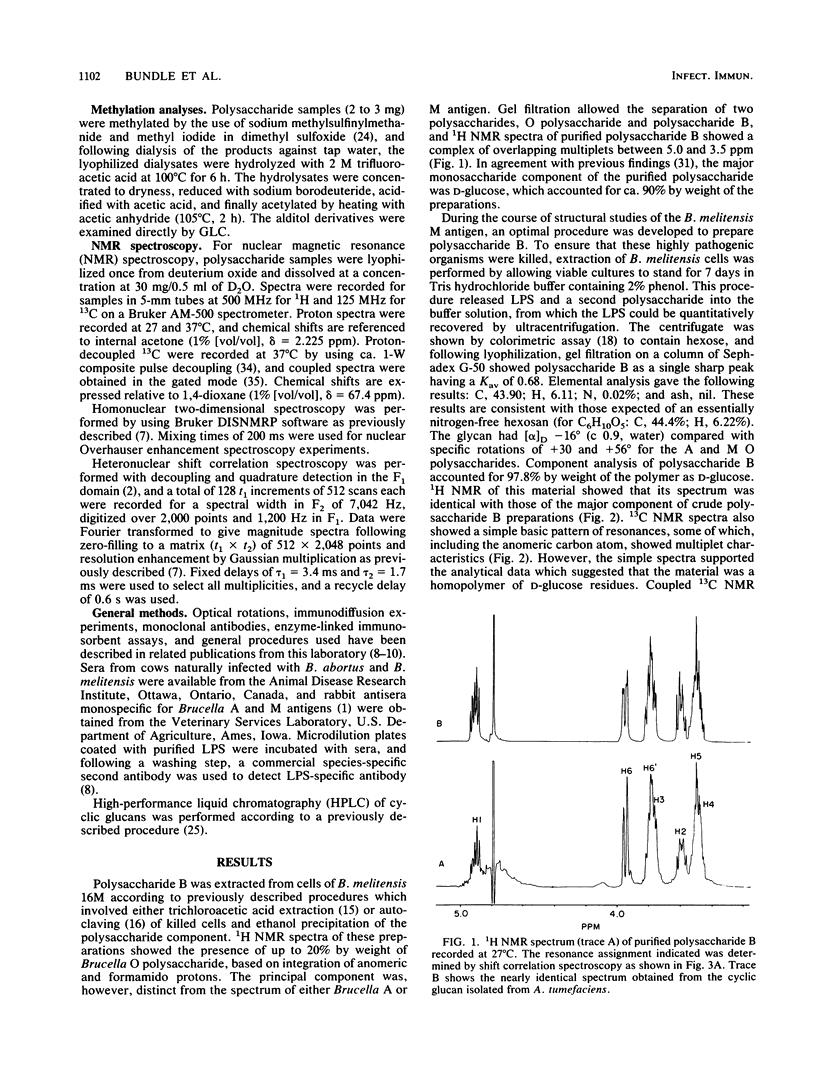
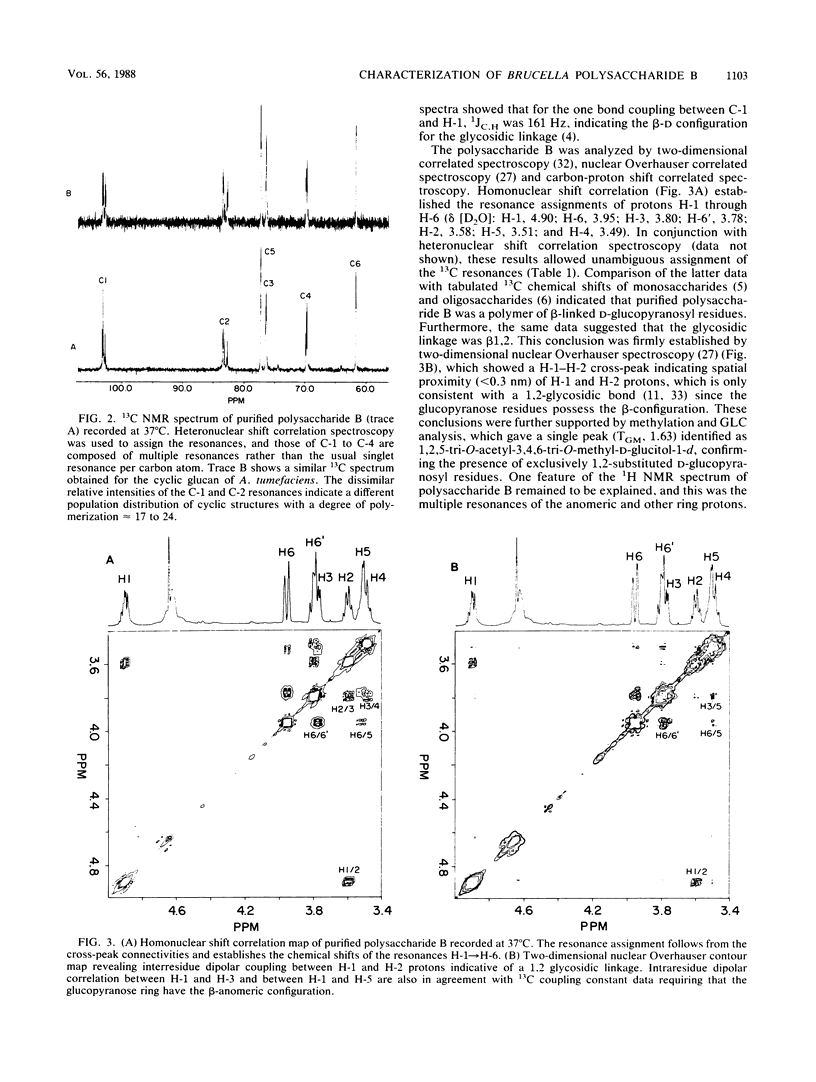
Polysaccharide B was extracted from Brucella melitensis 16M and from a rough strain of Brucella abortus 45/20 by autoclaving or trichloroacetic acid extraction of whole cells and by a new method involving mild leaching of cells. The material obtained by either of the established procedures was contaminated by O polysaccharide. The new leaching protocol eliminated this impurity and provided a pure glucan, which was regarded as polysaccharide B. This polysaccharide was found by high-performance liquid chromatography separations, chemical composition, methylation, and two-dimensional homo- and heteronuclear magnetic resonance experiments to be a family of nonreducing cyclic 1,2-linked polymers of beta-D-glucopyranosyl residues. The degree of polymerization varied between 17 and 24. Polysaccharide B was essentially identical to cyclic D-glucans produced by Rhizobia, Agrobacteria, and other bacterial species. Pure polysaccharide B did not precipitate with Brucella anti-A or anti-M serum and did not inhibit the serological reaction of Brucella A or M antigen with either bovine or murine monoclonal Brucella anti-A or anti-M serum. Previously described serological reactions of polysaccharide B preparations with Brucella anti-A and anti-M sera are related in this study to the presence in crude extracts of contaminants with the antigenic properties of Brucella lipopolysaccharide O polysaccharides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundle D. R., Cherwonogrodzky J. W., Perry M. B. Structural elucidation of the Brucella melitensis M antigen by high-resolution NMR at 500 MHz. Biochemistry. 1987 Dec 29;26(26):8717–8726. doi: 10.1021/bi00400a034. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984 Feb 15;139(1):195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Cherwonogrodzky J. W., Perry M. B., Bundle D. R. Identification of the A and M antigens of Brucella as the O-polysaccharides of smooth lipopolysaccharides. Can J Microbiol. 1987 Nov;33(11):979–981. doi: 10.1139/m87-172. [DOI] [PubMed] [Google Scholar]
- Dabrowski J., Hanfland P., Egge H., Dabrowski U. Immunochemistry of the Lewis-blood-group system: proton nuclear magnetic resonance study of plasmatic Lewis-blood-group-active glycosphingolipids and related substances. Arch Biochem Biophys. 1981 Aug;210(1):405–411. doi: 10.1016/0003-9861(81)90203-4. [DOI] [PubMed] [Google Scholar]
- Diaz R., Garatea P., Jones L. M., Moriyon I. Radial immunodiffusion test with a Brucella polysaccharide antigen for differentiating infected from vaccinated cattle. J Clin Microbiol. 1979 Jul;10(1):37–41. doi: 10.1128/jcm.10.1.37-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Levieux D. Rôle respiectif en sérologie de la brucellose bovine des antigènes et des immunoglobulines G 1 et G 2 dans les tests d'agglutination, de Coombs et au Rose Bengale ainsi que dans le phénomène de zone. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 6;274(10):1593–1596. [PubMed] [Google Scholar]
- Diaz R., Toyos J., Salvo M. D., Fernandez-Lago L., Alonso B., Moriyon I., Dorronsoro I. Studies on the polysaccharide B and native haptene of Brucella and Yersinia enterocolitica serotype 9. Dev Biol Stand. 1984;56:213–220. [PubMed] [Google Scholar]
- Diaz R., Toyos J., Salvó M. D., Pardo M. L. A simple method for the extraction of polysaccharide B from Brucella cells for use in the radial immunodiffusion test diagnosis of bovine brucellosis. Ann Rech Vet. 1981;12(1):35–39. [PubMed] [Google Scholar]
- Fernandez-Lago L., Moriyon I., Toyos J., Diaz R. Immunological identity of brucella native hapten, polysaccharide B, and yersinia enterocolitica serotype 9 native hapten. Infect Immun. 1982 Nov;38(2):778–780. doi: 10.1128/iai.38.2.778-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Jones L. M., Berman D. T., Moreno E., Deyoe B. L., Gilsdorf M. J., Huber J. D., Nicoletti P. Evaluation of a radial immunodiffusion test with polysaccharide B antigen for diagnosis of bovine brucellosis. J Clin Microbiol. 1980 Dec;12(6):753–760. doi: 10.1128/jcm.12.6.753-760.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Ernst R. R., Wüthrich K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem Biophys Res Commun. 1980 Jul 16;95(1):1–6. doi: 10.1016/0006-291x(80)90695-6. [DOI] [PubMed] [Google Scholar]
- L'vov V. L., Pluzhnikova G. N., Lapina E. B., Shashkov A. S., Askerova S. A. Molekuliarnaia priroda polisakharidnogo antigena brutsell (poli-B). Bioorg Khim. 1987 Aug;13(8):1070–1074. [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Speth S. L., Jones L. M., Berman D. T. Immunochemical characterization of Brucella lipopolysaccharides and polysaccharides. Infect Immun. 1981 Jan;31(1):214–222. doi: 10.1128/iai.31.1.214-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]


