Abstract
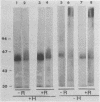
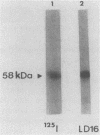
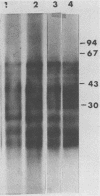
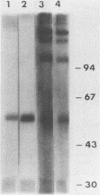
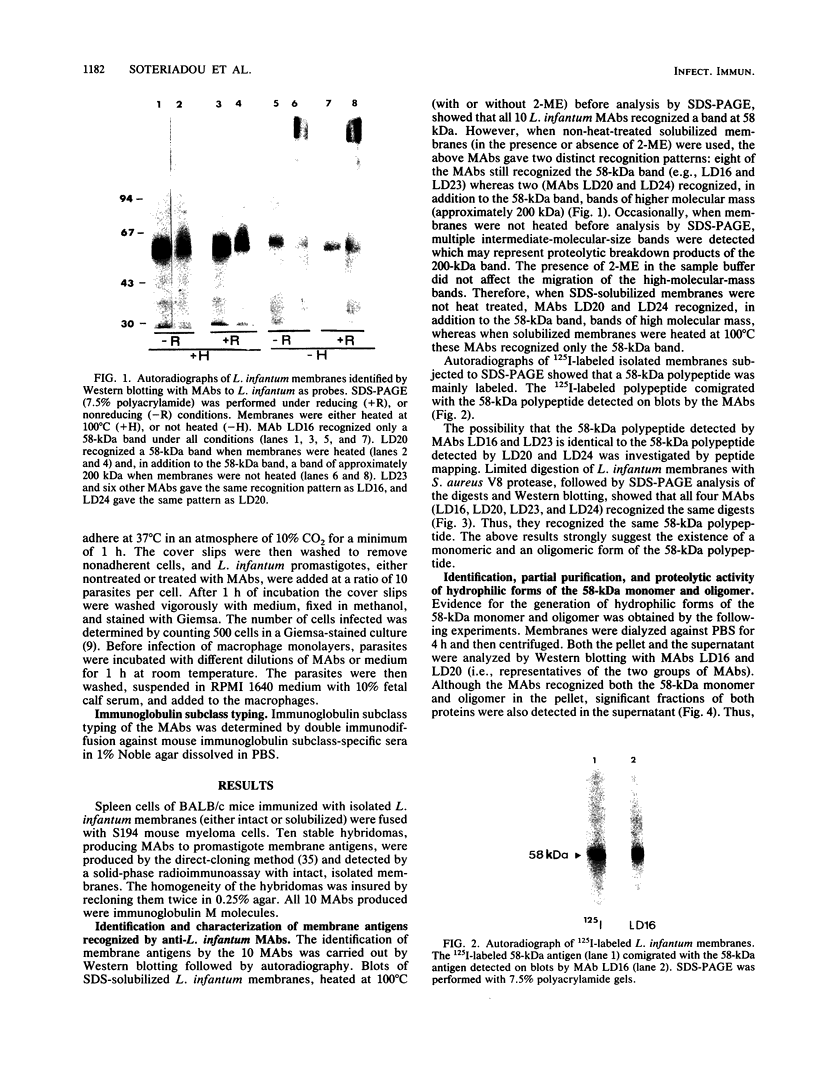
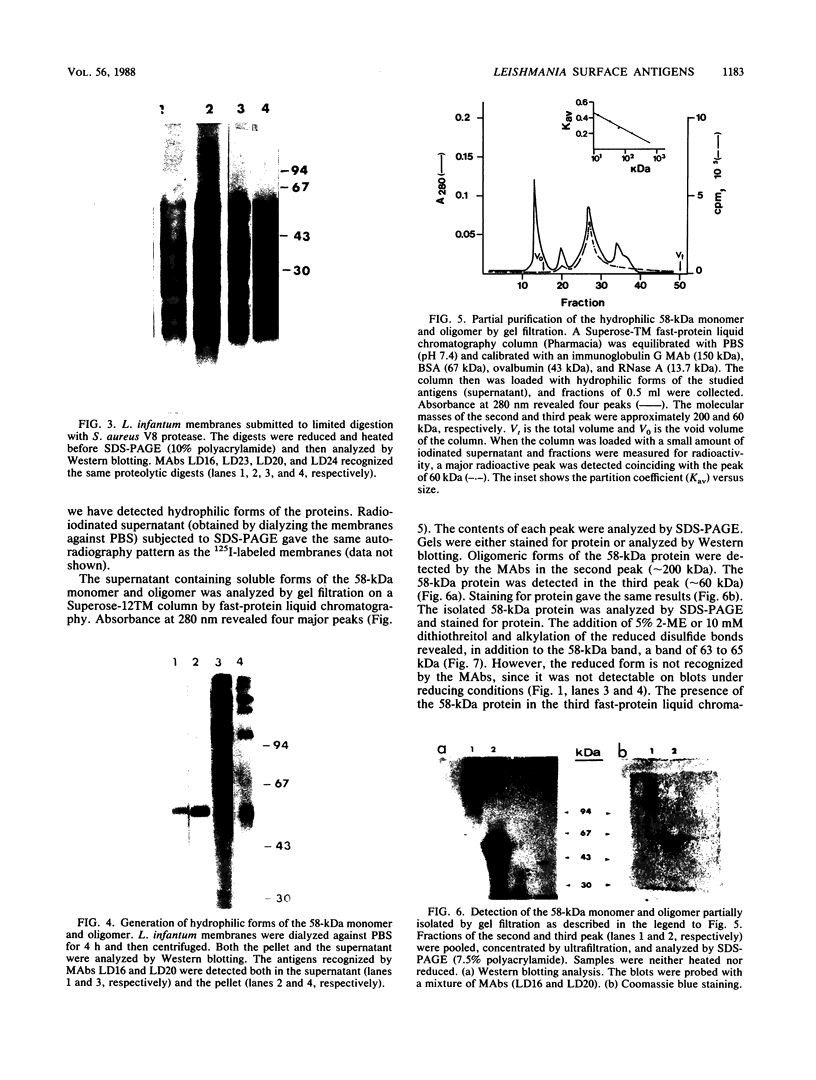
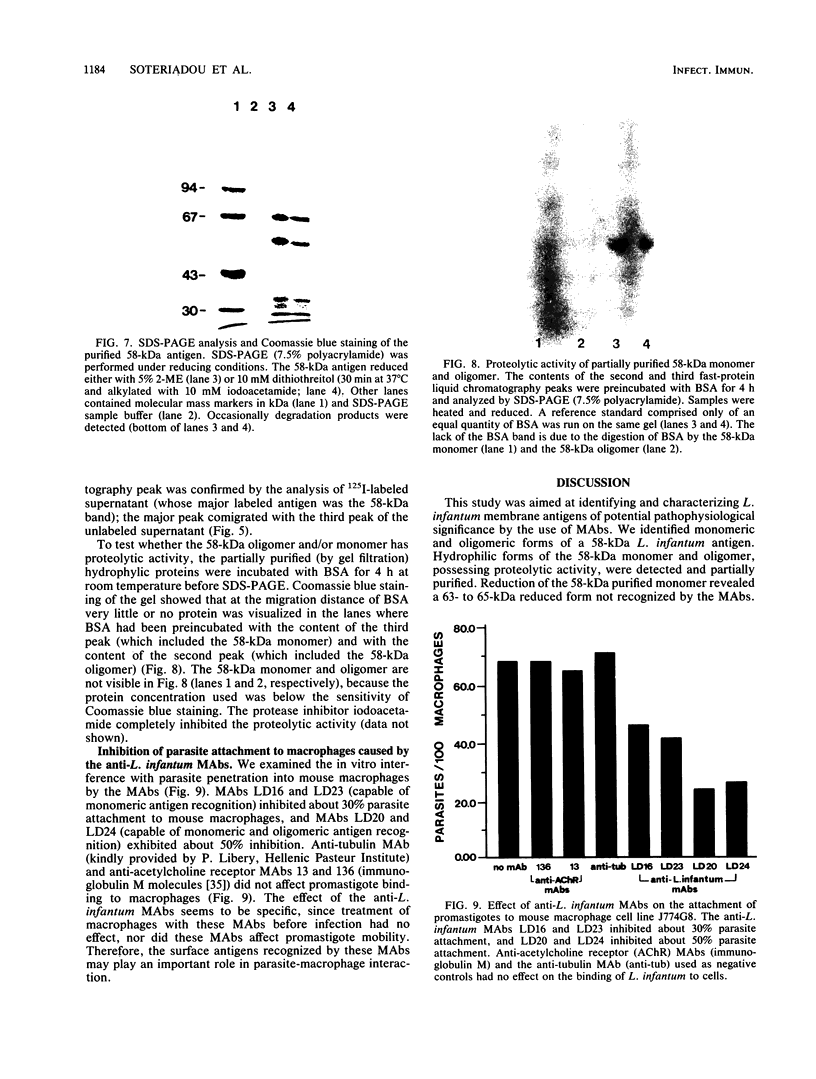
Ten monoclonal antibodies (MAbs) produced against isolated Leishmania infantum membranes were used as probes of L. infantum membrane antigens. Western blots of L. infantum membranes, sodium dodecyl sulfate solubilized and heated at 100 degrees C before analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, showed that all 10 MAbs recognized a band at 58 kilodaltons (kDa). However, when solubilized membranes were not heated, 2 of the 10 MAbs recognized, in addition to the 58-kDa band, bands of higher molecular weight. Limited digestion of heated or nonheated membranes showed that both groups of MAbs (i.e., not capable or capable of binding to the high-molecular-weight bands) recognized the same proteolytic digests. Hydrophilic forms of the above proteins, possessing proteolytic activity, were detected and isolated by gel filtration. Protein staining of the isolated monomer analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, under reducing and heating conditions, revealed incomplete reduction of the 58-kDa protein. The reduced form of the 58-kDa protein migrated at 63 to 65 kDa and was not recognized by the MAbs. These results suggest the existence of a monomeric and an oligomeric form of the 58-kDa antigen. The observed inhibition of Leishmania promastigote-macrophage binding caused by MAbs representative of the two groups (capable of oligomeric and/or monomeric antigen recognition) suggest that the 58-kDa monomer and oligomer play an important role in promastigote-macrophage interaction. We suggest that the 58-kDa L. infantum antigen is the major surface Leishmania antigen (p63) identified by others.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., David J. R., McMahon-Pratt D. In vivo protection against Leishmania mexicana mediated by monoclonal antibodies. J Immunol. 1983 Oct;131(4):1616–1618. [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Heat-modifiable envelope proteins of Bordetella pertussis. Infect Immun. 1986 Oct;54(1):109–117. doi: 10.1128/iai.54.1.109-117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R. J., Bordier C. Identification and purification of membrane and soluble forms of the major surface protein of Leishmania promastigotes. J Biol Chem. 1985 Dec 15;260(29):15504–15509. [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Cellular and molecular mechanisms of intracellular symbiosis in leishmaniasis. Int Rev Cytol Suppl. 1983;14:267–305. [PubMed] [Google Scholar]
- Chang K. P., Fong D. Cell biology of host-parasite membrane interactions in leishmaniasis. Ciba Found Symp. 1983;99:113–137. doi: 10.1002/9780470720806.ch7. [DOI] [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Isolation and partial characterization of surface membranes from Leishmania donovani promastigotes. J Protozool. 1980 May;27(2):176–182. doi: 10.1111/j.1550-7408.1980.tb04676.x. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is a protease. J Biol Chem. 1986 Jul 15;261(20):9098–9101. [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: Identification by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7366–7370. doi: 10.1073/pnas.79.23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P. R., Jaffe C. L., Dwyer D. M. Identification of cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect Immun. 1984 Feb;43(2):637–643. doi: 10.1128/iai.43.2.637-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt C. L., Slutzky G. M., de Ibarra A. A., Snary D. Monoclonal antibodies for serotyping Leishmania strains. J Clin Microbiol. 1983 Jul;18(1):191–193. doi: 10.1128/jcm.18.1.191-193.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Monoclonal antibodies specific for Leishmania tropica. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1983 Oct;131(4):1987–1993. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemesre J. L., Rizvi F. S., Afchain D., Sadigursky M., Capron A., Santoro F. Subspecies-specific surface antigens of promastigotes of the Leishmania donovani complex. Infect Immun. 1985 Oct;50(1):136–141. doi: 10.1128/iai.50.1.136-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon-Pratt D., Bennett E., David J. R. Monoclonal antibodies that distinguish subspecies of Leishmania braziliensis. J Immunol. 1982 Sep;129(3):926–927. [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Pratt D. M., Bennett E., Grimaldi G., Jaffe C. L. Subspecies- and species-specific antigens of Leishmania mexicana characterized by monoclonal antibodies. J Immunol. 1985 Mar;134(3):1935–1940. [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamouranis N., Schnur L. F., Garifallou A., Pateraki E., Sérié C. Leishmaniasis in Greece I. Isolation and identification of the parasite causing human and canine visceral leishmaniasis. Ann Trop Med Parasitol. 1984 Aug;78(4):363–368. doi: 10.1080/00034983.1984.11811833. [DOI] [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]