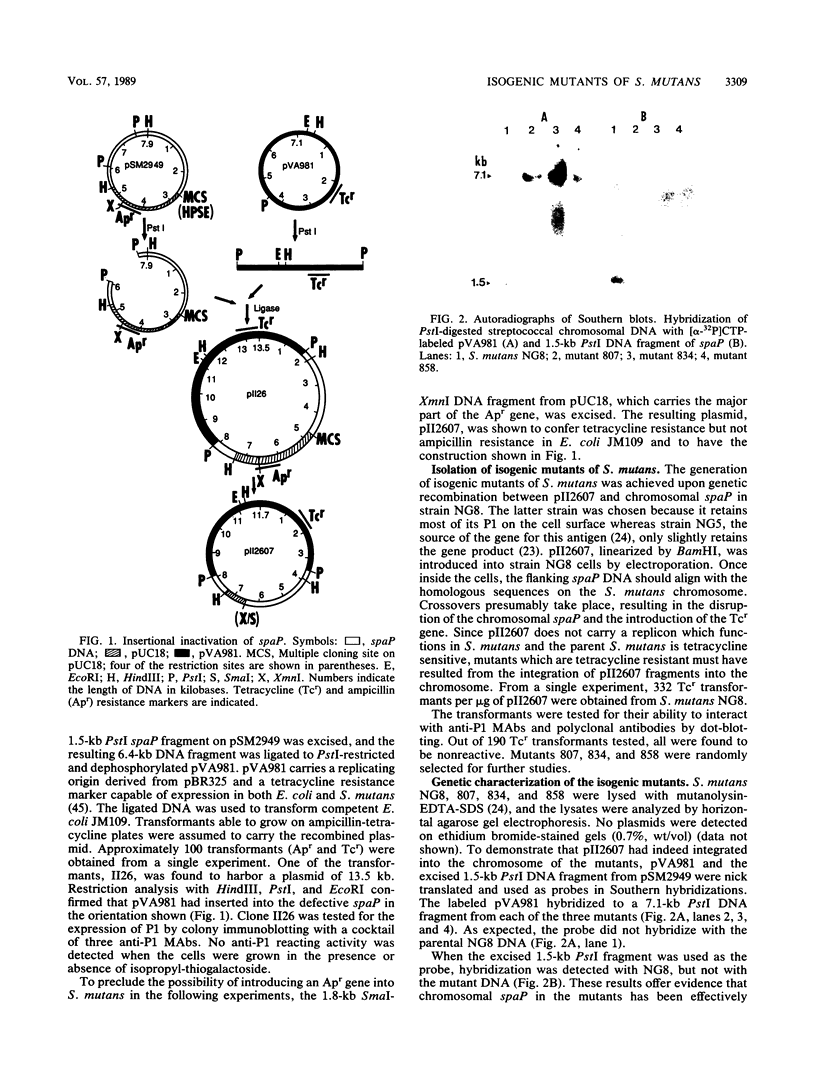
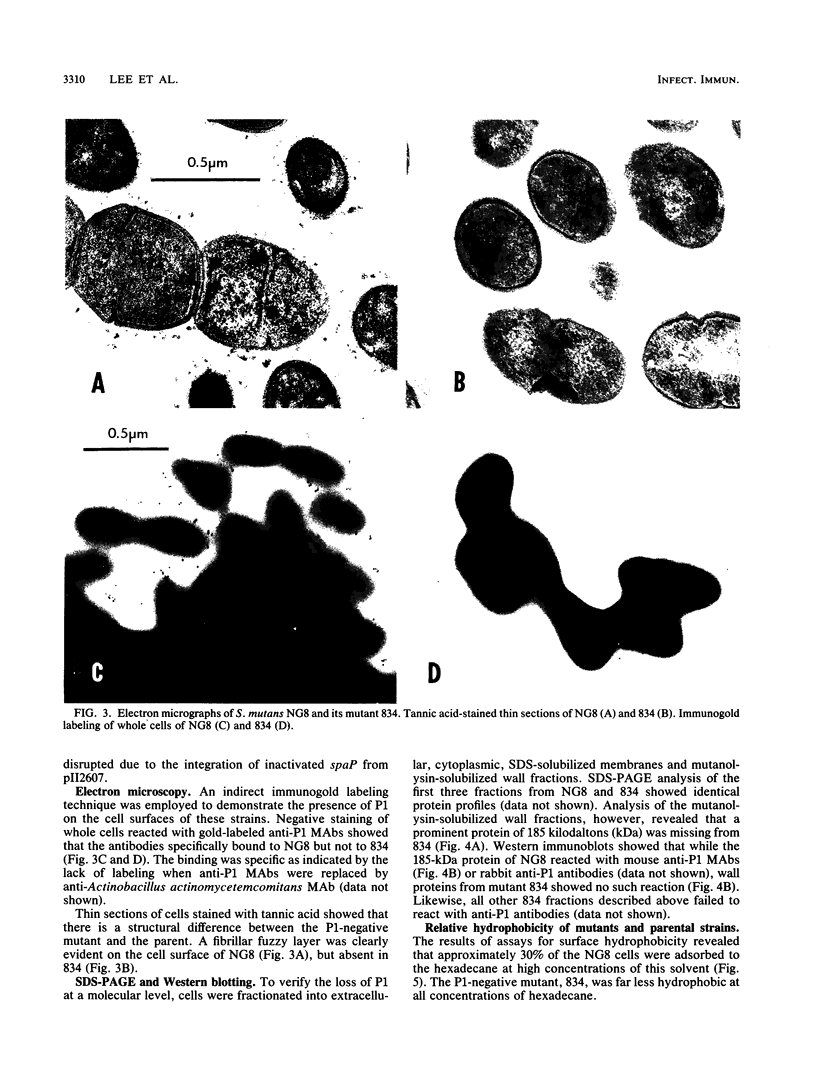
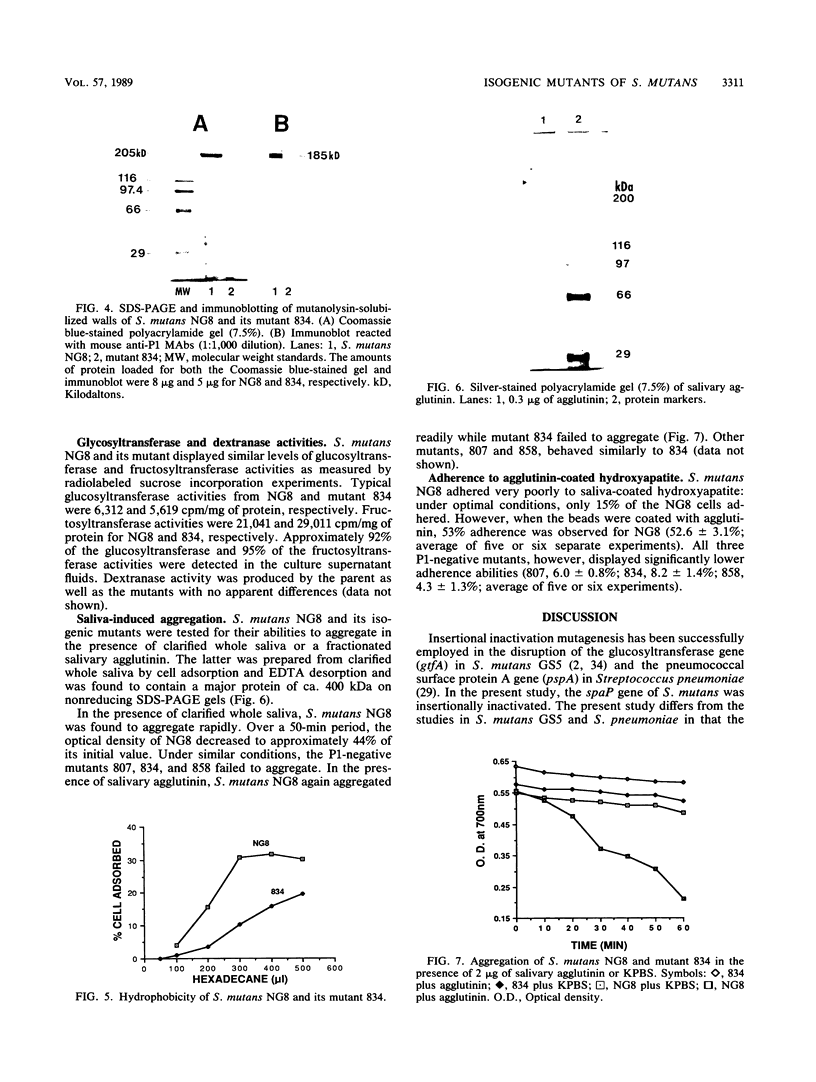
Abstract
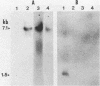
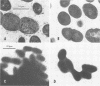
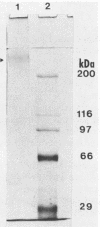
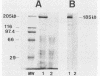
The gene (spaP) coding for the Streptococcus mutans major surface protein antigen P1 (or I/II) has been cloned into Escherichia coli (S. F. Lee, A. Progulske-Fox, and A. S. Bleiweis, Infect. Immun. 56:2114-2119, 1988). In the present study, this gene has been disrupted in vitro by insertional inactivation with pVA981, which carries a Tcr marker, and transformed into S. mutans NG8 (serotype c) by electroporation. Upon homologous recombination, the defective spaP was integrated into the genome as demonstrated by Southern hybridization analysis. One Tcr mutant, designated 834, selected by its nonreactivity with anti-P1 monoclonal antibodies, was found to lack the cell surface fuzzy layer which was clearly present on the parent cells. Analysis of extracellular fluids, sodium dodecyl sulfate-solubilized membranes, and cytoplasmic fractions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that 834 had protein profiles identical to the parent. However, a 185-kilodalton protein which reacts with anti-P1 antibodies was missing from the wall of 834, suggesting that spaP has been specifically inactivated. This mutant displayed levels of glucosyltransferase and fructosyltransferase activities similar to those of the parent. It was much less hydrophobic than the parent. S. mutans NG8 aggregated readily in the presence of clarified whole saliva or a high-molecular-weight salivary agglutinin. This strain also adhered to agglutinin-coated hydroxyapatite. The P1-negative mutants, however, did not display these two properties, suggesting that P1 may play a role in saliva-mediated aggregation and adherence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayakawa G. Y., Boushell L. W., Crowley P. J., Erdos G. W., McArthur W. P., Bleiweis A. S. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta R. G., Michalek S. M., Curtiss R., 3rd Analysis of the virulence of Streptococcus mutans serotype c gtfA mutants in the rat model system. Infect Immun. 1988 Feb;56(2):322–330. doi: 10.1128/iai.56.2.322-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985 Aug;49(2):459–462. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd 1984 Kreshover lecture. Genetic analysis of Streptococcus mutans virulence and prospects for an anticaries vaccine. J Dent Res. 1986 Aug;65(8):1034–1045. doi: 10.1177/00220345860650080101. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Davis C. A., Corner A. M., Lamont R. J., Leboy P. S., Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988 Sep;56(9):2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Thompson D. W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985 Mar;47(3):752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Handley P. S., Carter P. L., Wyatt J. E., Hesketh L. M. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect Immun. 1985 Jan;47(1):217–227. doi: 10.1128/iai.47.1.217-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. D., Handley P. S., Embery G. Surface fibrils may be responsible for the salivary glycoprotein-mediated aggregation of the oral bacterium Streptococcus sanguis. Arch Oral Biol. 1981;26(11):945–949. doi: 10.1016/0003-9969(81)90156-4. [DOI] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Barrett J. F., Clark-Curtiss J. E., Curtiss R., 3rd In vivo repackaging of recombinant cosmid molecules for analyses of Salmonella typhimurium, Streptococcus mutans, and mycobacterial genomic libraries. Infect Immun. 1986 Apr;52(1):101–109. doi: 10.1128/iai.52.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hardy L. N., Wicken A. J. Comparative studies on the protein profiles and hydrophobicity of strains of Streptococcus mutans serotype c. J Gen Microbiol. 1986 Sep;132(9):2541–2548. doi: 10.1099/00221287-132-9-2541. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. S., Yother J., Vijayakumar M., McGarry L., Guild W. R., Briles D. E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J Exp Med. 1987 Feb 1;165(2):381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., Ganeshkumar N., McBride B. C. Cell surface components of Streptococcus sanguis: relationship to aggregation, adherence, and hydrophobicity. J Bacteriol. 1985 Oct;164(1):255–262. doi: 10.1128/jb.164.1.255-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Powell Ian B., Achen Marc G., Hillier Alan J., Davidson Barrie E. A Simple and Rapid Method for Genetic Transformation of Lactic Streptococci by Electroporation. Appl Environ Microbiol. 1988 Mar;54(3):655–660. doi: 10.1128/aem.54.3.655-660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Macrina F. L. Molecular organization and expression of the gtfA gene of Streptococcus mutans LM7. Infect Immun. 1986 Oct;54(1):77–84. doi: 10.1128/iai.54.1.77-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundegren J., Arnold R. R. Differentiation and interaction of secretory immunoglobulin A and a calcium-dependent parotid agglutinin for several bacterial strains. Infect Immun. 1987 Feb;55(2):288–292. doi: 10.1128/iai.55.2.288-292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Beighton D., Cohen B. Immunisation of monkeys (Macaca fascicularis) with antigens purified from Streptococcus mutans. Br Dent J. 1982 Feb 2;152(3):81–84. doi: 10.1038/sj.bdj.4804751. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Peach S. L., Colman G., Cohen B. Antibody responses to antigens of Streptococcus mutans in monkeys (Macaca fascicularis) immunized against dental caries. J Gen Microbiol. 1983 Mar;129(3):865–875. doi: 10.1099/00221287-129-3-865. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian J. A., Cline M. L., Macrina F. L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders E. D., Lehner T. Separation and characterization of a protein antigen from cells of Streptococcus mutans. J Gen Microbiol. 1981 Feb;122(2):217–225. doi: 10.1099/00221287-122-2-217. [DOI] [PubMed] [Google Scholar]