Abstract
A novel single‐nucleotide deletion in exon 100 of the RYR1 gene, corresponding to deletion of nucleotide 14 510 in the human RyR1 mRNA (c14510delA), was identified in a man with malignant hyperthermia and in his two daughters who were normal for malignant hyperthermia. This deletion results in a RyR1 protein lacking the last 202 amino acid residues. All three subjects heterozygotic for the mutated allele presented with a prevalence of type 1 fibres with central cores, although none experienced clinical signs of myopathy. Expression of the truncated protein resulted in non‐functional RYR1 calcium release channels. Expression of wild‐type and RyR1R4836fsX4838 proteins resulted in heterozygotic release channels with overall functional properties similar to those of wild‐type RyR1 channels. Nevertheless, small differences in sensitivity to calcium and caffeine were observed in heterotetrameric channels, which also presented an altered assembly/stability in sucrose‐gradient centrifugation analysis. Altogether, these data suggest that altered RYR1 tetramer assembly/stability coupled with subtle chronic changes in Ca2+ homoeostasis over the long term may contribute to the development of core lesions and incomplete malignant hyperthermia susceptibility penetrance in individuals carrying this novel RYR1 mutation.
Mutations in the RYR1 gene, which encodes the type 1 sarcoplasmic reticulum Ca2+ release channel, are linked to skeletal muscle diseases including malignant hyperthermia (OMIM 145600), central core disease (CCD; OMIM 117000) and multi‐minicore disease (OMIM 255320).1,2,3 Malignant hyperthermia is an autosomal‐dominant pharmacogenetic disorder in which exposure to volatile anaesthetics and muscle relaxants results in life‐threatening whole‐body muscle contractions and hyperthermia. CCD and multi‐minicore disease are rare congenital myopathies, clinically characterised by hypotonia, delayed motor development, weakness of proximal muscles and histological lesions in skeletal muscle fibres. There is considerable clinical overlap between these two disorders, and each can present with either single or multiple core‐like structures characterised by the absence of mitochondria and oxidative enzyme activity. In some cases, specific mutations in the RYR1 gene have been shown to result in both malignant hyperthermia and CCD. In addition, core lesions in skeletal muscle fibres are also observed in >20% of individuals with malignant hyperthermia susceptibility (MHS), even in the absence of any clinical sign of myopathy.4
We report the identification of a novel RYR1 mutation (c14510delA) resulting in a premature truncation of the RYR1 protein, RyR1R4837fsX4839, in a person who experienced an episode of malignant hyperthermia during anaesthesia. The histological analysis of muscle biopsy specimens showed a clear prevalence of type 1 fibres (about 95%) and central core lesions in about 80% of fibres. This mutation was also found in two daughters of the proband, both of whom tested negative for malignant hyperthermia using a standard in vitro contracture test (IVCT), but presented the same histological changes in skeletal muscle fibres. However, neither the proband nor the two daughters exhibited myopathy, indicating that the central core lesions observed were sub‐clinical and do not adversely affect muscle function. Functional studies were performed to determine the potential relationship between the RyR1R4837fsX4839 mutation and the development of central core lesions in affected individuals.
Patients and methods
Patient evaluation, IVCT and RyR1 mutation screening
IVCT was performed on muscle biopsy specimens from vastus lateralis mounted in contracture chambers and exposed to increasing concentrations of halothane or caffeine according to the protocol of the European Malignant Hyperthermia Group.5 Histological analysis was performed on frozen muscle sections by staining with haematoxylin–eosin or Gomori's modified trichrome reaction. Parallel sections were stained for cytocrome c oxidase, succinate dehydrogenase and nicotinamide adenine dinucleotide–tetrazolium reductase. Genomic DNA was extracted from peripheral blood leucocytes following standard methods. RYR1 genomic structure and intron boundary sequences were deduced from the Homo sapiens chromosome 19 genomic contig NT_011109 and cDNA sequence NM_000540.1. Polymerase chain reaction primers, denaturing high‐performance liquid chromatography screening and DNA sequencing condititions have been described previously.6
Measurement of cytosolic free Ca2+ concentrations in HEK293 cells expressing wild‐type and truncated RyR1 proteins
Wild‐type rabbit RyR1 cDNA was cloned into the pcDNA3 vector (Invitrogen, Paisley, UK). The ClaI‐XbaI fragment from the rabbit RyR1 cDNA was subcloned into the pBluescript vector (Stratagene, La Jolla, California, USA) to perform site‐directed mutagenesis using two oligonucleotides containing the deletion of rabbit RyR1 nucleotide 14507, corresponding to nucleotide 14510 of human RyR1 cDNA. Owing to differences between rabbit and human RyR1 sequences, the human deletion mutant R4837fsX4839 corresponds to rabbit R4836fsX4838. Human embryonic kidney (HEK)293 cells were transfected using the Lipofectamine Plus method (Invitrogen) following the manufacturer's instruction. Cells were analysed 24–48 h after transfection. Methods for microsomal protein preparation, western blot analysis and [3H]ryanodine binding have been described previously.7 Sucrose density gradient analysis of 3‐[3‐(chloramidopropyl)dimethylammonio]‐1‐propanesulphonate‐solubilised ryanodine receptors was performed as described by Lai et al.8
For Ca2+ release experiments, HEK293 cells were loaded with 5 μM fura‐2‐AM (acetoxymethyl ester derivative of fura‐2; Calbiochem, Darmstadt, Germany) in Krebs‐Ringer‐HEPES medium. Images were acquired with a digital charge coupled device camera (Princeton Instruments, Ottobrunn, Germany), and the fura‐2 fluorescence emission ratio (340/380 excitation, 515 emission) was analysed using the Metafluor software (Universal Imaging Corporation, Sunnyvale, California, USA).
Excitation–contraction coupling measurements after expression in dyspedic myotubes
Myotubes from RyR1‐knockout (dyspedic) mice were prepared as described previously.9 After 4–7 days of the initial plating of myoblasts, nuclei of individual dyspedic myotubes were microinjected with cDNAs encoding CD8 (0.1 μg/μl) and 0.5 μg/μl wild‐type RyR1, 0.5 μg/μl RyR1R4836fsX4838 (HM) or 0.25 μg/μl wild‐type RyR1 with 0.25 μg/μl RyR1R4836fsX4838 (HT). Electrically evoked and agonist‐induced Ca2+ release was measured in intact myotubes loaded with indo‐1 AM (acetoxymethyl ester derivative of indo‐1; Molecular Probes, Eugene, Oregon, USA) as described previously.10,11 The whole‐cell patch‐clamp technique was used to simultaneously measure the voltage dependence of L‐type Ca2+ currents (L‐currents) and intracellular Ca2+ transients in expressing myotubes11,12,13 using an internal solution containing (in mM) 145 Cs‐aspartate, 10 CsCl, 0.1 Cs2EGTA, 1.2 MgCl2, 5 Mg‐ATP, 10 HEPES and 0.2 K5‐fluo‐4, pH 7.4, and an external solution containing (in mM) 145 TEA‐Cl, 10 CaCl2, 10 HEPES, pH 7.4. Inward L‐currents were elicited by 200‐ms test pulses of variable amplitude from a holding potential of −80 mV delivered immediately after a prepulse protocol used to inactivate T‐type Ca2+ channels (1 s to −30 mV followed by 25 ms to −50 mV). Peak L‐current amplitude (I) was normalised to total cell capacitance (pA/pF), plotted as a function of membrane potential, and fitted according to equation 1:
I = Gmax(Vm−Vrev)/(1+exp) (VG1/2−Vm)/kG)
where Gmax is the maximal L‐channel conductance, Vm the test potential, Vrev the extrapolated reversal potential, VG1/2 the voltage for half‐maximal activation of Gmax and kG a slope factor. Ca2+ transients were simultaneously recorded during each test pulse and are reported as ΔF/F, where F represents baseline fluo‐4 fluorescence immediately before depolarisation and ΔF the fluorescence change from baseline. The magnitude of voltage‐gated Ca2+ transients (ΔF/F) was determined by fitting the data according to equation 2:
ΔF/F = (ΔF/F)max/(1+exp) (VF1/2−Vm)/kF)
where (ΔF/F)max is the calculated maximal change in fluo‐4 fluorescence, VF1/2 the voltage for half‐maximal activation of (ΔF/F)max, and kF a slope factor.
Results and discussion
Clinical history, IVCT and molecular genetic analyses
The proband is a 77‐year‐old man with an unremarkable clinical history, with the exception of a malignant hyperthermia crisis with hyperthermia and tachycardia during surgical procedures under general anaesthesia carried out with the administration of volatile anaesthetic agents and succinylcholine (malignant hyperthermia rank 4 according to Larach et al14). MHS was confirmed after IVCT performed according to the protocol of the European Malignant Hyperthermia Group, a test with a diagnostic sensitivity of 99% and an overall false‐positive or false‐negative error probability in the diagnosis of <1%5 (our unpublished observations). Histological analysis was performed on frozen muscle sections stained with haematoxylin–eosin and Gomori's modified trichrome reaction. Parallel sections were stained for cytochrome c oxidase, succinate dehydrogenase and nicotinamide adenine dinucleotide–tetrazolium reductase. Staining identified central core lesions in 80% of fibres and, a clear prevalence of type 1 fibres (about 95%) was detected by staining for myosin ATPase at pH 9.4 (data not shown). No evidence of inflammatory reaction or involvement of connective tissue was observed. However, in contrast with that observed for patients with central core disease, motor activities were not impaired and the patient was essentially asymptomatic, with only mild muscle hypotonia and muscle weakness. Serum creatine kinase concentration was normal (64 IU/l). Five healthy relatives were also tested by IVCT (individuals III3, III4, III5, IV1 and IV2; fig 1A). All were found to be negative for malignant hyperthermia. However, histological analysis of muscle biopsy specimens of individuals III3 and III5 also showed the presence of central core lesions in >70% of the fibres and a prevalence of type 1 fibres (about 95%), although no clinical signs were observed even after follow‐up evaluations.
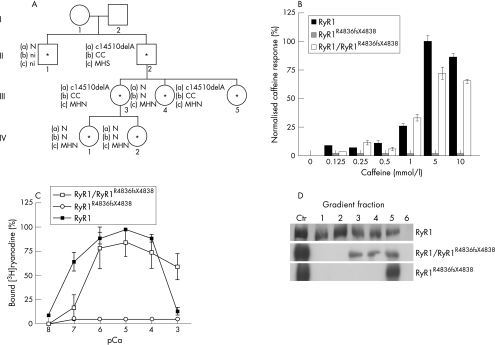
Figure 1 Identification and functional characterisation of the RyR1R4836fsX4838 mutation. (A) The family pedigree. The proband is individual II2. Asterisks indicate individuals who were included in the mutation analysis. (a) Presence (c14510delA) or absence (N) of the mutation. (b) Histological results: central cores (CC), normal (N) and not investigated individuals (ni). (c) In vitro contracture test results. (B) Caffeine responses of human embryonic kidney (HEK)293 cells expressing recombinant RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels. Release was normalised to the maximum Ca2+ release at 5 mM caffeine. Bars indicate mean (standard error of the mean (SEM)) of the percentage of Ca2+ release increments induced by different caffeine concentrations. n = 759 cells for RyR1/RyR1R4836fsX4838; n = 601 cells for RyR1; n = 600 cells for RyR1R4836fsX4838. (C) Ca2+ dependence of [3H]ryanodine binding to microsomal fractions of HEK293 cells with RyR1, RyR1R4836fsX4838 or RyR1/RyR1R4836fsX4838. Mean values obtained from three independent experiments are plotted. Bars indicate the mean (SEM) of 3[H]ryanodine binding. (D) Sucrose density‐gradient analysis of wild‐type RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels expressed in HEK293 cells. As a positive control for RyR1 migration, microsomal vesicles obtained from rabbit skeletal muscle (5 μg) and HEK293 cells expressing RyR1R4836fsX4838 (middle, 30 μg) or RyR1/RyR1R4836fsX4838 (bottom, 30 μg) channels were used (Ctr lane). Fraction 1 represents the bottom of the gradient. MHN, malignant hyperthermia negative; MHS, malignant hyperthermia susceptibility.
Screening of all the 106 exons of the RYR1 gene showed that the propositus (II2) and his two daughters (III3 and III5) were heterozygotic for a single‐base deletion in exon 100, corresponding to deletion of nucleotide 14510 in the human RyR1 mRNA (c.14510delA). This deletion was not detected in 100 normal individuals. To confirm the expression of the mutated allele in the skeletal muscle of affected individuals, RyR1 cDNA was cloned from RNA extracted from muscle biopsy specimens of relevant individuals, and nucleotide sequence analysis showed an expression level equivalent to wild‐type and mutated alleles (data not shown). This deletion alters the open reading frame where amino acids Gln4837 and Leu4838 are replaced by Arg and Trp residues, and an opal stop codon appears five nucleotides downstream of the deletion. Thus, the mutated protein lacks the last 202 amino acid residues in the C‐terminal region of the channel, including the putative selectivity filter and the final two transmembrane domains. According to convention, the human deletion mutant is referred to from here on as R4837fsX4839.15 Interestingly, the individuals that carry the deletion presented with a high incidence of central core lesions in the absence of myopathy, although no lesions were detected in individuals lacking the mutant allele.
Functional properties of RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels expressed in HEK293 cells
To evaluate the caffeine sensitivity of RyR1 channels carrying the R4836fsX4838 mutation, HEK293 cells were loaded with fura‐2 and stimulated with caffeine concentrations from 0.125 to 10 mmol/l (fig 1B). Caffeine activated calcium release from wild‐type RyR1‐expressing HEK293 cells with an EC50 = 3 mmol/l. However, HEK293 cells expressing RyR1R4836fsX4838 alone completely lacked caffeine‐induced calcium release even up to concentrations as high as 10 mmol/l. Cells expressing equal amounts of wild‐type and mutant RyR1 exhibited caffeine‐induced calcium release that showed caffeine sensitivity comparable to that observed in cells expressing wild‐type RyR1 channels alone (EC50 = 3 mmol/l). However, the peak magnitude of calcium release observed on stimulation with 5 and 10 mM caffeine was significantly (p<0.05 and p<0.001, respectively) reduced after coexpression of wild‐type and RyR1R4836fsX4838 channels (72.36% and 65.47% reduction at 5 and 10 mM caffeine, respectively).
The calcium dependence of [3H]ryanodine binding was investigated in microsomes prepared from HEK293 cells expressing wild‐type RyR1, RyR1R4836fsX4838 or heterotetrameric RyR1/RyR1R4836fsX4838 channels (fig 1C). Wild‐type RyR1 channels exhibited a characteristic bimodal calcium dependence of [3H]ryanodine binding (fig 1C, closed squares). Heterotetrameric RyR1/RyR1R4836fsX4838 channels reached maximal binding at pCa values between 6 and 4, but unlike wild‐type RyR1 channels, binding remained high (only about 20% reduction) even at pCa = 3. Interestingly, a similar reduction in calcium‐dependent (and magnesium‐dependent) inhibition of ryanodine binding is also observed for other mutations in RyR1 that result in MHS.16 Homozygotic expression of RyR1R4836fsX4838 channels exhibited negligible [3H]ryanodine binding at all Ca2+ concentrations (pCa 8–3), indicating that the truncation either results in a constitutively closed channel or a marked disruption in the ryanodine‐binding site (fig 1C, open circles).
Sucrose‐density gradient analysis of recombinant RyR1, RyR1R4836fsX4838 and RyR1/RyR1R4836fsX4838 channels expressed in HEK293 cells
The C‐terminal region of either RyR or InsP3R channels has been proposed to contain sequences responsible for channel tetramerisation.17,18,19,20 To verify whether lack of the C‐terminal domain in RyR1R4836fsX4838 truncated channels might affect assembly of protein subunits into tetramers, the sucrose density sedimentation profile of recombinant channels was analysed on 5–20% linear sucrose gradients as described previously.8,17,18 As fig 1D shows, most of the wild‐type RyR1 channels were detected in fraction 2 of the gradients, which corresponds to the sedimentation profile of tetrameric RyR channels.8 By contrast, in gradients loaded with extracts from HEK293 cells expressing RyR1R4836fsX4838, RyR immunoreactivity was predominant in fraction 5, which corresponds to the region of the gradient where monomeric, unassembled, RyR1 proteins migrate.8 This suggests that RyR1R4836fsX4838 subunits do not readily assemble into stable tetramers. The sucrose sedimentation profile of extracts from HEK293 cells expressing RyR1/RyR1R4836X4838 recombinant proteins showed that immunoreactivity for heterotetrameric channels was present in fractions intermediate to those of RyR1R4836fsX4838 and wild‐type channels, with higher levels of protein in fractions 3–5, thus suggesting that these complexes are likely to be less stable than homotetrameric wild‐type RyR1 channels, similar to that observed for C‐terminal truncations of the InsP3R.18
Effects of the RyR1R4836fsX4838 truncation on excitation–contraction coupling RyR1 in skeletal myotubes
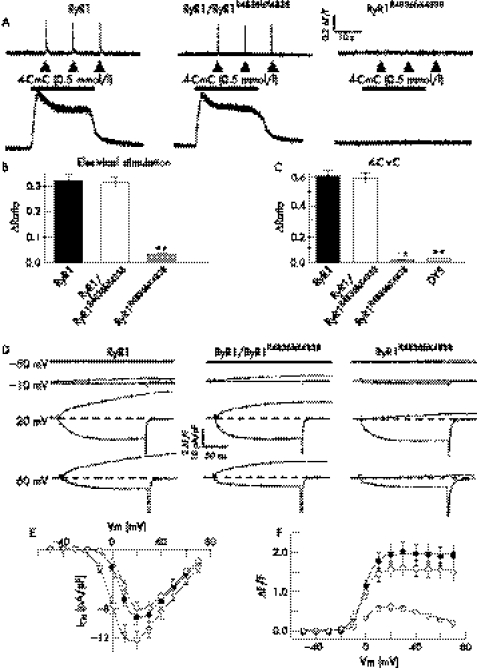
To investigate the effect of the RyR1R4836fsX4838 truncation on RyR1 function in a skeletal muscle environment, we expressed wild‐type RyR1 alone, RyR1R4836fsX4838 alone, or wild‐type RyR1 with RyR1R4836fsX4838 in dyspedic myotubes (fig 2). Calcium release in response to electrical stimulation (fig 2A, upper traces) and 4‐chloro‐m‐cresol (fig 2A, lower traces) were similar in RyR1‐expressing and RyR1/RyR1R4836fsX4838‐expressing myotubes. In addition, caffeine concentration–response analysis found a similar magnitude (ΔRatio = 0.49 (0.03) and 0.53 (0.03), respectively) and sensitivity (EC50 = 4.1 (0.7), n = 35, and 5.4 (0.7), n = 39, respectively) for caffeine‐induced calcium release in RyR1‐expressing and RyR1/RyR1R4836fsX4838‐expressing myotubes. However, electrically evoked (fig 2B) and agonist‐induced (fig 2C) calcium release were essentially absent in myotubes expressing RyR1R4836fsX4838 proteins alone.
Figure 2 Effects of the RyR1R4836fsX4838 truncation on bidirectional dihydropyridine receptor–RyR1 coupling. (A–C) Electrically evoked and ligand‐induced Ca2+ release. (A) Representative indo‐1 traces obtained from intact myotubes expressing wild‐type RyR1 alone, RyR1R4836fsX4838 alone or RyR1/RyR1R4836fsX4838 after electrical stimulation (top traces) or application of 500 μM 4‐chloro‐m‐cresol (CmC) (lower traces). Average peak magnitude of electrically‐evoked (B) and 4‐CmC‐induced (C) Ca2+ release. **p<0.001 compared with wild‐type RyR1. DYS, uninjected dyspedic myotubes. (D) Representative voltage‐gated Ca2+ transients (upper traces) and L‐type Ca2+ currents (L‐currents; lower traces) recorded in response to 200‐ms depolarising pulses to the indicated membrane potentials (left). Average voltage dependence of L‐currents (E) and peak Ca2+ release (F) from the sarcoplasmic reticulum for dyspedic myotubes expressing RyR1 alone (closed circles), RyR1R4836fsX4838 alone (open triangles) and RyR1/RyR1R4836fsX4838 (open circles). The mean values (standard error) of the parameters describing the voltage dependence of Ca2+ current (I–V) and Ca2+ release (ΔF/F) obtained by fitting each myotube within a group separately are given in table 1.
Excitation–contraction coupling in the skeletal muscle involves a bidirectional signalling interaction between the dihydropyridine receptor (DHPR) and RyR1 such that the DHPR triggers RyR1 activation (orthograde coupling) and the presence of RyR1 enhances or modifies the calcium channel properties of the DHPR.13,21 We characterised effects of the RyR1 truncation on orthograde and retrograde DHPR–RyR1 coupling in whole‐cell patch‐clamp experiments of naive dyspedic myotubes and dyspedic myotubes expressing wild‐type RyR1 alone, RyR1R4836fsX4838 alone or wild‐type RyR1 with RyR1R4836fsX4838 (fig 2D–F and table 1). Retrograde enhancement of DHPR L‐channel activity was similarly restored after expression in dyspedic myotubes of wild‐type RyR1 alone, RyR1R4836fsX4838 alone or wild‐type RyR1 with RyR1R4836fsX4838 (fig 2E and table 1). The full restoration of DHPR L‐channel activity after expression of RyR1R4836fsX4838 indicates that the truncated protein was readily expressed, targeted to the sarcoplasmic reticulum–sarcolemmal junction, and functionally interacted with DHPRs within these junctions. Although the magnitude and voltage dependence of calcium release from the sarcoplasmic reticulum were similar in RyR1‐expressing and RyR1R4836fsX4838‐expressing myotubes, depolarisation‐induced Ca2+ transients were small and exhibited a bell‐shaped voltage dependence after expression of RyR1R4836fsX4838 alone (fig 2F and table 1). The absence of agonist‐induced (fig 2C) and voltage‐gated (fig 2B, F) calcium release from the sarcoplasmic reticulum in homotypic RyR1R4836fsX4838‐expressing myotubes is consistent with the truncation abolishing calcium permeation of the release channel.
Table 1 Parameters of fitted I–V and ΔF/F−V curves.
| n | I–V | ΔF/F−V | ||||||
|---|---|---|---|---|---|---|---|---|
| Gmax (nS/nF) | kG | VG1/2 (mV) | Vrev (mV) | (ΔF/F)max | VF1/2 (mV) | kF | ||
| RYR1 | 26 | 181 (14) | 5.1 (0.2) | 9.4 (0.8) | 77.7 (2.4) | 2.0 (0.2) | −2.3 (1.0) | 4.1 (0.4) |
| RYR1/RYR1R4836fsX4838 | 23 | 156 (15) | 5.3 (0.2) | 12.2 (0.9) | 78.8 (2.6) | 1.7 (0.2) | −1.3 (1.8) | 3.9 (0.3) |
| RYR1R4836fsX4838 | 27 | 214 (17) | 5.0 (0.2) | 2.2 (1.3)* | 81.8 (2.7) | – | – | – |
| DYS | 12 | 37 (4)* | 7.3 (0.6)* | 18 (1.4)* | 69.5 (5) | – | – | – |
Values represent mean (SE) for the number of experiments indicated in column n. Parameters for the voltage dependence of Ca2+ current (I–V) and Ca2+ release (ΔF/F) were obtained by fitting myotubes within each group separately to the appropriate equation (I–V, Eq 1; ΔF/F, Eq 2) as described in the Patients and methods section.
*p<0.001 compared with wild‐type RyR1.
DYS, dyspedic; F, baseline fluro‐4 fluorescence immediately before depolarization; ΔF, fluorescence change from baseline; (ΔF/F)max, maximal change in relative fluo‐4 fluorescence; Gmax, maximal L‐channel conductance; –V, current–voltage; kF, slope factor forΔF/F; kG, slope factor for I–V; VF1/2, voltage for half‐maximal activation of (ΔF/F)max; VG1/2, voltage for half‐maximal activation of Gmax; Vrev, L‐channel reversal potential; –, no response.
In conclusion, we propose that subtle changes in Ca2+ release of human heteromeric RyR1/RyR1R4837fsX4839 channels, probably due to the reduced stability/assembly of these channels, may predispose individuals to MHS, although with only a low degree of penetrance (eg, MHS status of the proband but not his two carrier daughters). This variable degree of penetrance for MHS might arise from differences between individuals with regard to wild‐type:mutant stoichiometry, presence or absence of other modifying genes, or a combination of both. Nevertheless, reduced tetramer formation/stability coupled with subtle chronic changes in Ca2+ homoeostasis over the long term may lead to the development of central core lesions and altered fibre composition observed in muscle biopsy specimens of individuals heterozygotic for the RyR1R4837fsX4839 allele.
Acknowledgements
We thank Dr Paul D Allen for providing access to the dyspedic mice used in this study, and Linda Groom for excellent technical assistance.
Abbreviations
CCD - central core disease
DHPR - dihydropyridine receptor
HEK - human embryonic kidney
IVCT - in vitro contracture test
MHS - malignant hyperthermia susceptibility
Footnotes
Funding: This work was supported by a research grant from the National Institutes of Health (AR44657 to RTD) and by grants from Telethon (number GGP02168), EU grant (HPRN‐CT‐2002‐00331) and from MIUR/PRIN 2003 to VS.
Competing interests: None declared.
References
- 1.MacLennan D H, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk R G, Frodis W, Britt A, Worton R G. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 1990343559–561. [DOI] [PubMed] [Google Scholar]
- 2.Monnier N, Romero N B, Lerale J, Nivoke Y, Qi D, MacLennan D H, Fardeau M, Lunardi J. An autosomal dominant congenital myopathy with cores and rods is associated with a neomutation in the RYR1 gene encoding the skeletal muscle ryanodine receptor. Hum Mol Genet 200092599–2608. [DOI] [PubMed] [Google Scholar]
- 3.Monnier N, Ferreiro A, Marty I, Labarre‐Vila A, Mezin P, Lunardi J. A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi‐minicore disease congenital myopathy with ophtalmoplegia. Hum Mol Genet 2003121171–1178. [DOI] [PubMed] [Google Scholar]
- 4.Monnier N, Kozak‐Ribbens G, Krivosic‐Horber R, Nivoke Y, Qi D, Kraev N, Loke J, Sharma P, Tegazzin V, Figarella‐Branger D, Romero N, Mezin P, Bendahan D, Payen J F, Depret T, MacLennan D H, Lunardi J. Correlations between genotype and pharmacological, histological, functional, and clinical phenotypes in malignant hyperthermia susceptibility. Hum Mutat 200526413–425. [DOI] [PubMed] [Google Scholar]
- 5.Ording H. In vitro contracture test for the diagnosis of malingant hyperthermia following the protocol of the European MH Group: Results of testing patients surviving fulminant MH and unrelated low‐risk subjects. Acta Anaesthesiol Scand 199741955–966. [DOI] [PubMed] [Google Scholar]
- 6.Galli L, Orrico A, Lorenzini S, Censini S, Falciani M, Covacci A, Tegazzin V, Sorrentino V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum Mutat 200627830–832. [DOI] [PubMed] [Google Scholar]
- 7.Rossi D, Simeoni I, Micheli M, Bootman M, Lipp P, Allen P D, Sorrentino V. RyR1 and RyR3 isoforms provide distinct intracellular Ca2+ signals in HEK293 cells. J Cell Sci 20021152497–2504. [DOI] [PubMed] [Google Scholar]
- 8.Lai F A, Misra M, Xu L, Smith A, Meissner G. The ryanodine receptor‐Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. J Biol Chem 198926416776–16785. [PubMed] [Google Scholar]
- 9.Avila G, O'Brien J J, Dirksen R T. Excitation‐contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc Natl Acad Sci USA 2001984215–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila G, Dirksen R T. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol 2001118277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell K M, Yamaguchi N, Meissner G, Dirksen R T. Calmodulin binding to the 3614–3643 region of RyR1 is not essential for excitation‐contraction coupling in skeletal myotubes. J Gen Physiol 2002120337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirksen R T, Avila G. Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1. Biophys J 2004873193–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avila G, Dirksen R T. Functional impact of the ryanodine receptor on the skeletal muscle L‐type Ca2+ channel. J Gen Physiol 2002115467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larach M G, Localio A R, Allen G C, Denborough M A, Ellis F R, Gronert G A, Kaplan R F, Muldoon S M, Nelson T E, Ording H. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 199480771–779. [DOI] [PubMed] [Google Scholar]
- 15.den Dunnen J T, Antonarakis S E. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000157–12. [DOI] [PubMed] [Google Scholar]
- 16.Yang T, Anh Ta T, Pessah I N, Allen P D. Functional defects in six ryanodine receptor isoform‐1 (RyR1) mutations asociated with malignant hyperthermia and their impact on skeletal excitation‐contraction coupling. J Biol Chem 200327825722–25730. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Tripathy A, Lu X, Meissner G. Evidence for a role of C‐terminal amino acid residues in skeletal muscle Ca2+ release channel (ryanodine receptor) function. FEBS Lett 1997412223–226. [DOI] [PubMed] [Google Scholar]
- 18.Galvan D L, Mignery G A. Carboxy‐terminal sequences critical for Inositol 1,4,5‐trisphosphate receptor subunit assembly. J Biol Chem 200227748248–48260. [DOI] [PubMed] [Google Scholar]
- 19.Stewart R, Zissimopoulos S, Lai F A. Oligomerization of the cardiac ryanodine receptor C‐terminal tail. Biochem J 2003376795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George C H, Jundi H, Thomas N L, Scoote M, Walters N, Williams A J, Lai F A. Ryanodine receptor regulation by intramolecular interaction between cytoplasmic and transmembrane domains. Mol Biol Cell 2004152627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakai J, Dirksen R T, Nguyer H T, Pessah I N, Beam K G, Allen P D. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 199638072–75. [DOI] [PubMed] [Google Scholar]