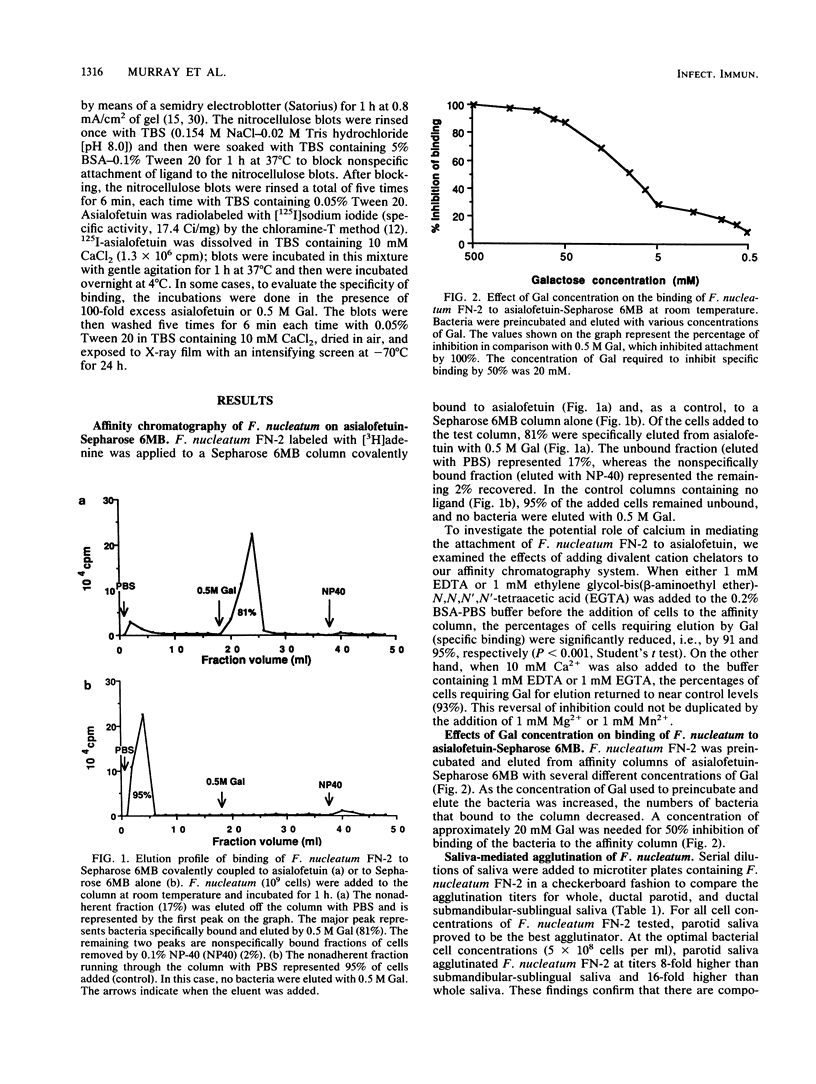
Abstract
A previous study has suggested that Fusobacterium nucleatum FN-2 contains a galactose-binding protein (lectin) on the cell surface (P. A. Murray, V. Matarese, C. I. Hoover, and J. R. Winkler, FEMS Microbiol. Lett. 40:123-127, 1987). In the present study, the molecular specificity and size of this lectin were investigated by several techniques. Whole-cell affinity chromatography with asialofetuin covalently coupled to Sepharose 6MB demonstrated that 81% of 3H-labeled F. nucleatum were specifically eluted by 0.5 M galactose. Specific binding was calcium dependent and did not occur in the presence of calcium chelators. Binding was inhibited by preincubation with galactose. Agglutination of human parotid saliva by F. nucleatum was also inhibited by galactose and its structural analogs. Inhibition by lactose was 2 times that of galactose, inhibition by p-aminophenyl galactosides was 4 times that of galactose, and inhibition by asialoglycopeptides was 100 times that of galactose. Similar inhibition results were obtained for hemagglutination of neuraminidase-treated erythrocytes. These findings suggest that the binding specificity of F. nucleatum FN-2 is more complex than simply the recognition of the monosaccharide galactose. This is consistent with the concept that lectins considered identical in terms of monosaccharide specificity can recognize fine differences in more complex structures. To identify the specific bacterial component(s) involved in galactose recognition, proteins of F. nucleatum FN-2 were separated on a 4 to 11% gradient sodium dodecyl sulfate slab gel, transferred to nitrocellulose paper to renature bacterial binding sites, and then incubated with 125I-labeled asialofetuin. Autoradiographs of the nitrocellulose revealed a band at a range of Mr 300,000 to 330,000 which was not present when the blots were preincubated with galactose. These data support the concept that F. nucleatum FN-2 possesses a lectin that recognizes galactose and galactose-containing substrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H., Nowak T. P. Limulus polyphemus agglutinin (limulin). Methods Enzymol. 1978;50:302–305. doi: 10.1016/0076-6879(78)50032-3. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Sandberg A. L., Mergenhagen S. E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984 Mar;63(3):393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Hawley C. E. Hemagglutinating activity of Fusobacterium nucleatum. Infect Immun. 1977 Jan;15(1):230–238. doi: 10.1128/iai.15.1.230-238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Mongiello J. R., Burger B. W. Haemagglutination inhibition and aggregation of Fusobacterium nucleatum by human salivary mucinous glycoproteins. Arch Oral Biol. 1979;24(7):483–489. doi: 10.1016/0003-9969(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffler H., Barondes S. H. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J Biol Chem. 1986 Aug 5;261(22):10119–10126. [PubMed] [Google Scholar]
- Mongiello J. R., Falkler W. A., Jr Sugar inhibition of oral Fusobacterium nucleatum haemagglutination and cell binding. Arch Oral Biol. 1979;24(7):539–545. doi: 10.1016/0003-9969(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Reddy M. S., Tabak L. A., Bergey E. J. Preparation of a sialic acid-binding protein from Streptococcus mitis KS32AR. Infect Immun. 1986 Aug;53(2):359–365. doi: 10.1128/iai.53.2.359-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Prakobphol A., Murray P. A., Fisher S. J. Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Jul;164(1):5–11. doi: 10.1016/0003-2697(87)90359-9. [DOI] [PubMed] [Google Scholar]
- Smoot C. N., Falkler W. A., Jr Attachment of serum non-antibody glycoproteins to the Fusobacterium nucleatum found in periodontal disease. Arch Oral Biol. 1981;26(11):859–864. doi: 10.1016/0003-9969(81)90143-6. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



