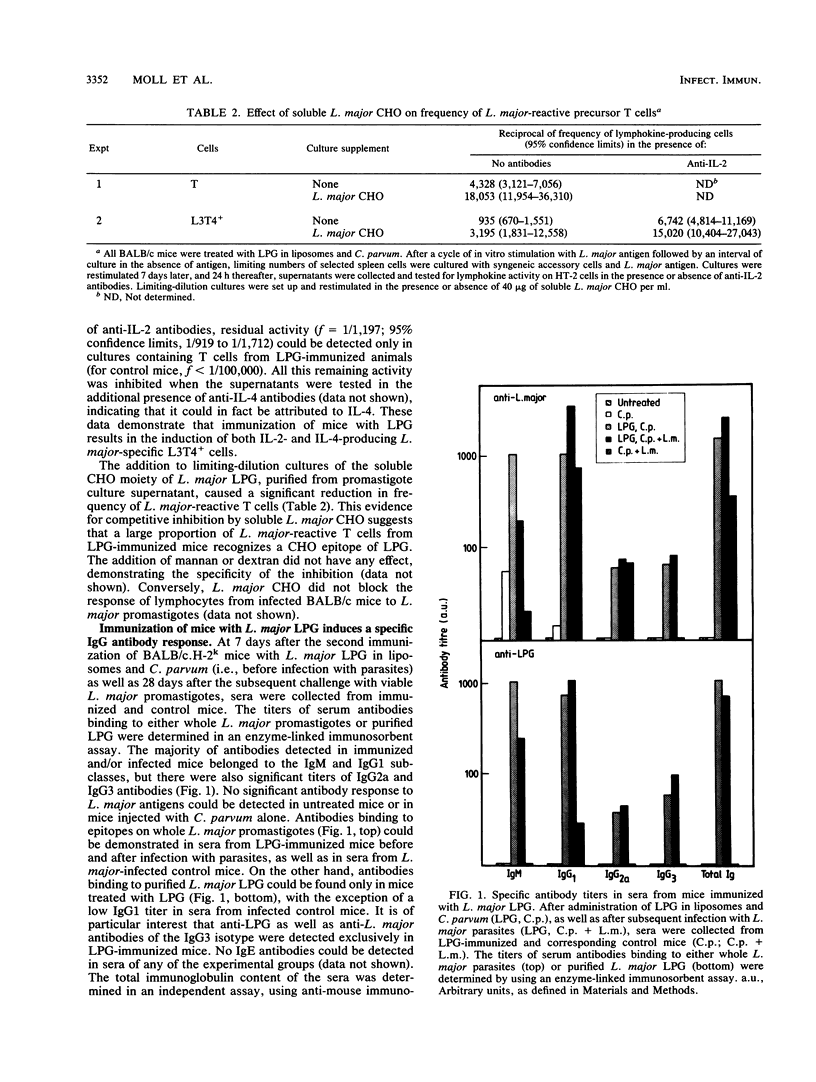
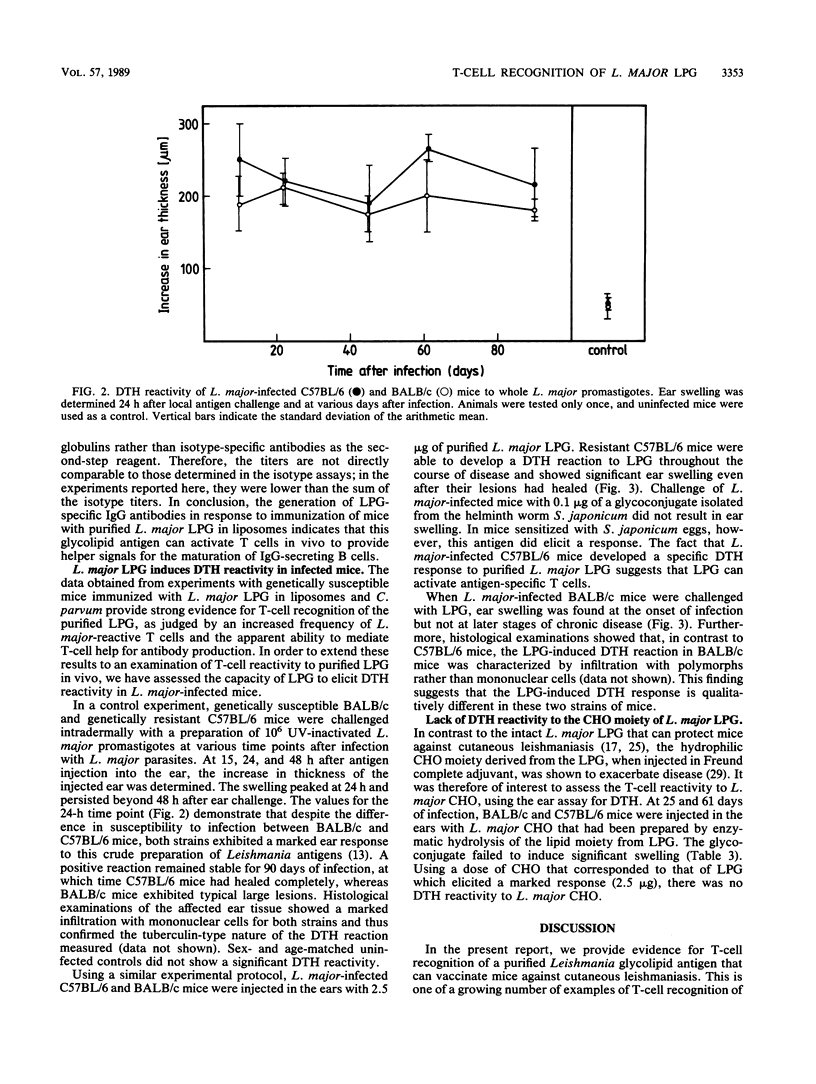
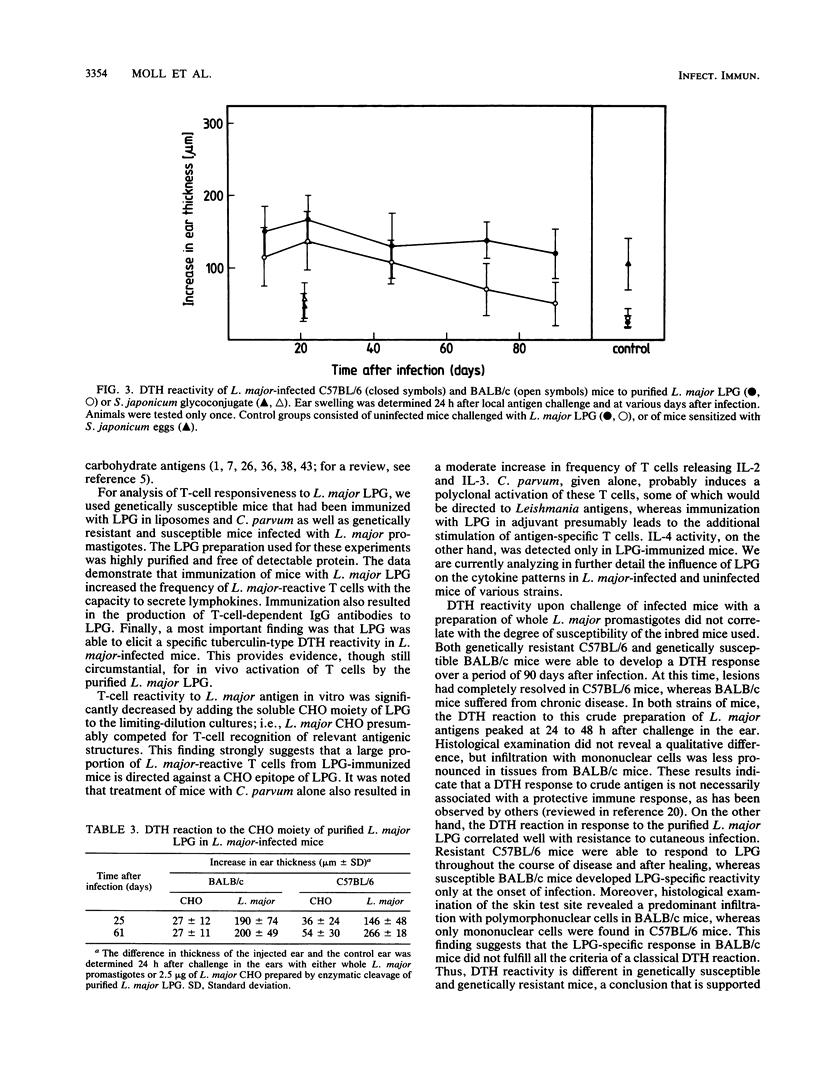
Abstract
We have previously reported that a Leishmania major lipophosphoglycan (LPG), given with killed Corynebacterium parvum as an adjuvant, can vaccinate mice against cutaneous leishmaniasis. In order to analyze whether T cells are able to recognize this important parasite antigen, we have studied both humoral and cellular immune responses to L. major LPG that had been isolated from promastigotes by sequential solvent extraction and hydrophobic chromatography. The data show that immunization of mice with highly purified LPG induced an increase in frequency of L. major-reactive T cells and the production of immunoglobulin G antibodies to LPG. Furthermore, genetically resistant mice infected with L. major were able to develop a specific delayed-type hypersensitivity response in the ear to L. major LPG. These findings strongly suggest that T cells can recognize and respond to glycolipid antigens, in this case a host-protective Leishmania LPG, even though such antigens appear not to be potent T-cell stimulators in mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAER H., CHAPARAS S. D. TUBERCULIN REACTIVITY OF A CARBOHYDRATE COMPONENT OF UNHEATED BCG CULTURE FILTRATE. Science. 1964 Oct 9;146(3641):245–247. doi: 10.1126/science.146.3641.245. [DOI] [PubMed] [Google Scholar]
- Bordier C., Etges R. J., Ward J., Turner M. J., Cardoso de Almeida M. L. Leishmania and Trypanosoma surface glycoproteins have a common glycophospholipid membrane anchor. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5988–5991. doi: 10.1073/pnas.83.16.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. The promastigote surface protease of Leishmania. Parasitol Today. 1987 May;3(5):151–153. doi: 10.1016/0169-4758(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Immunization against tuberculosis: what kind of vaccine? Infect Immun. 1988 Nov;56(11):2769–2773. doi: 10.1128/iai.56.11.2769-2773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossell R. A., Bray R. S., Alexander J. The correlation between delayed hypersensitivity, lymphocyte activation and protective immunity in experimental murine leishmaniasis. Parasite Immunol. 1987 Jan;9(1):105–115. doi: 10.1111/j.1365-3024.1987.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Garner R. E., Befidi-Mengue R. N. Mannan as an antigen in cell-mediated immunity (CMI) assays and as a modulator of mannan-specific CMI. Infect Immun. 1989 Mar;57(3):693–700. doi: 10.1128/iai.57.3.693-700.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Frommel D., Ogunkolade B. W., Vouldoukis I., Monjour L. Vaccine-induced immunity against cutaneous leishmaniasis in BALB/c mice. Infect Immun. 1988 Apr;56(4):843–848. doi: 10.1128/iai.56.4.843-848.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Liew F. Y. Cell-mediated immunity in experimental cutaneous Leishmaniasis. Parasitol Today. 1986 Oct;2(10):264–270. doi: 10.1016/0169-4758(86)90135-3. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Hale C., Howard J. G. Immunologic regulation of experimental cutaneous leishmaniasis. V. Characterization of effector and specific suppressor T cells. J Immunol. 1982 Apr;128(4):1917–1922. [PubMed] [Google Scholar]
- Locksley R. M., Heinzel F. P., Sadick M. D., Holaday B. J., Gardner K. D., Jr Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Molecular control of granulocyte and macrophage production. Prog Clin Biol Res. 1985;191:323–337. [PubMed] [Google Scholar]
- Mitchell G. F., Curtis J. M., Handman E., McKenzie I. F. Cutaneous leishmaniasis in mice: disease patterns in reconstituted nude mice of several genotypes infected with Leishmania tropica. Aust J Exp Biol Med Sci. 1980 Oct;58(5):521–532. doi: 10.1038/icb.1980.54. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Moll H., McConville M. J., Spithill T. W., Kidane G. Z., Samaras N., Elhay M. J. Resistance and susceptibility of mice to Leishmania major: a view from Melbourne. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):738–743. doi: 10.1016/s0769-2625(87)80029-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. The glycoconjugate derived from a Leishmania major receptor for macrophages is a suppressogenic, disease-promoting antigen in murine cutaneous leishmaniasis. Parasite Immunol. 1986 May;8(3):255–263. doi: 10.1111/j.1365-3024.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Moll H., Scollay R., Mitchell G. F. Resistance to cutaneous leishmaniasis in nude mice injected with L3T4+ T cells but not with Ly-2+ T cells. Immunol Cell Biol. 1988 Feb;66(Pt 1):57–63. doi: 10.1038/icb.1988.7. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Powderly W. G., Pier G. B., Markham R. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. V. Generation of bactericidal T cells in nonresponder mice. J Immunol. 1987 Apr 1;138(7):2272–2277. [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Robertsson J. A., Svenson S. B., Lindberg A. A. Salmonella typhimurium infection in calves: delayed specific skin reactions directed against the O-antigenic polysaccharide chain. Infect Immun. 1982 Aug;37(2):737–748. doi: 10.1128/iai.37.2.737-748.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987 Jul 1;139(1):221–227. [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Tiu W. U., Davern K. M., Garcia E. G., Moll H., Mitchell G. F. Monoclonal antibodies reacting with Schistosoma japonicum eggs and their target epitopes. Acta Trop. 1989 Mar;46(2):75–92. doi: 10.1016/0001-706x(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]


