Abstract
Assessment strategies are an important component in game theoretical models of contests. Strategies can be either based on one’s own abilities (self assessment) or on the relative abilities of two opponents (mutual assessment). Using statistical methodology that allows discrimination between assessment types, we examined contests in the jumping spider Phiddipus clarus. In this species, aggressive interactions can be divided into ‘pre-contact’ and ‘contact’ phases. Pre-contact phases consist of bouts of visual and vibratory signaling. Contact phases follow where males physically contact each other (leg fencing). Both weight and vibratory signaling differences predicted winners with heavier and more actively signaling males winning more contests. Vibratory behaviour predicted pre-contact phase duration, with higher signaling rates and larger differences between contestants leading to longer pre-contact interaction times. Contact phase duration was predicted most strongly by the weight of losing males relative to that of winning males, suggesting that P. clarus males use self-assessment in determining contest duration. While a self-assessment strategy was supported, our data suggest a secondary role for mutual assessment (“partial mutual assessment”). After initial contest bouts, male competitors changed their behaviour. Pre-contact and contact phase durations were reduced while vibratory signaling behaviour in winners was unchanged. In addition, only vibratory signaling differences predicted winners in subsequent bouts suggesting a role of experience in determining contest outcomes. We suggest that the rules and assessment strategies males use can change depending on experience and that assessment strategies are likely a continuum between self- and mutual assessment.
Introduction
Many game theoretical models have been developed to examine how animal contests are resolved. These models fall into one of two main categories based on the type of assessment that occurs during the contest: mutual- or self assessment. In mutual assessment models, individuals assess their own resource holding potential (RHP) relative to their opponent. The assessment of RHP can occur based on characters that correlate with fighting ability (reviewed in Hsu et al., 2006) including body size, weight, energy reserves, weaponry, and acoustic signals (Garland & Kelly, 2006; Taylor et al., 2001; Hoefler, 2007; Enquist & Leimer, 1990; Prenter et al., 2006; Briffa & Elwood, 2000; Morrell et al., 2005; Bridge et al., 2000; Davidson & Wilkinson, 2004; Mason, 1996). In mutual assessment models, contest duration is predicted to be negatively correlated with the relative RHP of contestants, as closely matched contestants take longer to perceive RHP differences (Enquist & Leimer, 1987; Enquist & Leimer, 1983; Enquist & Leimer, 1990). The ability to assess differences in RHP has been incorporated into several game theory models of animal conflicts (Morrell et al., 2005; Parker & Rubenstein, 1981; Hammerstein & Parker, 1982; Maynard Smith & Parker, 1976; Mesterton-Gibbons et al., 1996; Enquist & Leimer, 1983; Enquist & Leimer, 1990; Leimar et al., 1991). Empirical studies have supported several of these models (Stuart-Fox, 2006; Enquist & Jakobsson, 1986; Enquist & Leimer, 1990; Jennions & Backwell, 1996; Jensen & Yngvesson, 1998; Bridge et al., 2000; Kokko & López-Sepulcre, 2007; but see Taylor et al., 2001; Draud et al., 2004; Leimar et al., 1991; Keeley & Grant, 1993).
In contrast, in self-assessment models, individuals do not assess the quality of their rivals, but instead males set a threshold based on their own ability and decisions are made solely on a male’s own ability/reserves. Individuals with relatively smaller RHP reach their threshold first and essentially “give up” sooner than their opponent, independent of their opponent’s RHP. As in mutual assessment models, in self-assessment models contest duration is negatively correlated with the RHP difference between the contestants (Taylor et al., 2001; Mesterton-Gibbons et al., 1996; Payne, 1998). Several recent empirical studies have supported the self assessment hypothesis (Taylor et al., 2001; Prenter et al., 2006; Morrell et al., 2005; Bridge et al., 2000; Jennings et al., 2004; Garland & Kelly, 2006).
As both self- and mutual assessment models predict a negative relationship between contest duration and RHP asymmetry (Taylor et al., 2001) distinguishing between the two models requires careful examination of contest dynamics (Taylor et al., 2001; Morrell et al., 2005; Prenter et al., 2006; Gammell & Hardy, 2003). Using a simulation model, Taylor and Elwood (2001) outlined how it is possible to distinguish between self and mutual assessment mechanisms by comparing the direction of the correlation coefficients between the RHP of winners and losers (or larger and smaller rivals). In both assessment models, loser RHP should correlate positively with duration. In self assessment models, winner RHP should also correlate positively with duration but more weakly than the positive correlation between loser RHP and duration (Taylor et al., 2001). In contrast, in mutual assessment models, winner RHP should be negatively correlated with contest duration, with approximately the same strength as the positive correlation between loser RHP and duration (Taylor et al., 2001).
In later studies, Prenter et. al (2006) and Morrell et. al (2005) modified predictions associated with both mutual and self assessment models to include situations of “partial mutual assessment”. In these cases, contest duration is driven most strongly by self assessment mechanisms, but animals are able to gather information about opponents and use this information to modify their decisions (Prenter et al., 2006). As more information becomes available, the relationship between winner RHP and duration is predicted to shift from slightly positive to negative values (Prenter et al., 2006). Alternatively, Morrell et. al (2005) proposed that cumulative assessment games (Payne 1998) best fit some situations suggestive of “partial mutual assessment”. Cumulative assessment games are similar to individual threshold models as individuals have a threshold of costs they are willing to pay, but differ from these models in that contestants with greater RHP inflict higher costs and/or lower-RHP contestants amass costs more quickly than their opponents (Briffa & Elwood, 2000; Payne, 1998; Taylor et al., 2001; Prenter et al., 2006; Morrell et al., 2005). Morrell et al. (2005) went on to suggest that by examining the direction of the standardized partial regression (β) coefficients in a multiple regression model, one could detect the presence of cumulative assessment mechanisms (Morrell et al., 2005; Taylor et al., 2001).
Jumping spiders have been used in several studies examining male contests (Taylor et al., 2001; Hoefler, 2007; Pollard et al., 1987; Faber & Baylis, 1993; Wells, 1988; Cross et al., 2007; Cross et al., 2006). When two males meet, they usually enter into stereotyped displays consisting of visual and tactile signals (Taylor et al., 2001; Pollard et al., 1987). In addition to visual displays, some jumping spiders have been described as producing substrate borne (vibratory) signals (Pollard et al., 1987; Elias et al., 2003; Elias et al., 2005; Elias et al., 2006; Wynne-Edwards, 2001; Gwynne & Dadour, 1985; Taylor et al., 2001). It is yet unknown whether substrate borne signals are important in aggressive contexts.
Phiddipus clarus is a common jumping spider in mid-successional fields throughout North America and has a very restricted breeding season, mating in mid July and ovipositing in August (Roach 1988, Hoefler 2007). Individuals build silken nests (retreats) in rolled-up leaves on plants and return to the same nests throughout their lifetime (Hoefler, 2007). During the breeding season, adult male P. clarus visit and guard immature female nests over a period of several weeks (Hoefler 2007; Elias, Kasumovic & Punzalan personal observation). During this time males engage in repeated contests with numerous rivals for access to female’s nests. Due to the short lifespan of males (Elias personal observation; Hoefler 2007), successful mate guarding is of critical importance in ensuring reproductive success. Males prefer larger females and larger males are more likely to win contests, leading to size-assortative pairing (Hoefler, 2007).
The aim of this study was to determine (1) the presence and importance of substrate-borne signals in male contests, (2) the assessment mechanisms used in contests, and (3) the factors that decide contest outcomes.
Methods
Spiders
We collected adult and penultimate (one moult from maturity) male and female P. clarus from the Koffler Scientific Reserve at Jokers Hill, King City, Ontario, Canada in June and July 2006. Each spider was held in the laboratory individually in 2×2×3 cm cages in a 12:12hr light:dark cycle and kept in visual isolation from one another. We cut the bottom off a 1.5ml plastic Eppendorf tube and placed a tube in each container to give spiders a substrate to build nests. Spiders were fed several small crickets (Acheta domesticus) and flies (Drosophila hydeii) approximately twice each week. We housed individuals for at least four days prior to any experiments to allow individuals to acclimate to laboratory conditions.
Experimental Setup
At least four days prior to experiments, males were weighed and numbered. Males were then anesthetized with CO2 and two dots of non-toxic paint (Luminous paint, BioQuip Products, Inc) were placed on each male’s abdomen (opisthosoma) to allow individual identification of males during contests (both live and in videotapes). We ensured that males recovered from the anesthetic by verifying that males fed on prey after the procedure.
A plastic cylinder (12 cm in diameter 9 cm high) was used as the experimental arena. Petroleum jelly was placed on the inside of the cylinder wall to prevent spiders from crawling out of the arena and an opaque paper ring was placed around the outside of the cylinder to prevent unwanted visual distractions. A piece of graph paper, cut to fit inside the cylinder, was used as the arena floor. We replaced the graph paper every two trials to prevent the build up of any chemical cues. A Frezzi Minifill light was used to illuminate the arena as we videotaped the contest from above (Navitar Zoom 7000 lens, JAI CV-S3200 CCD camera, Sony DVCAM DSR-20 digital VCR). We recorded substrate vibrations produced during interactions using a laser doppler vibrometer (LDV) (Polytec OFV 3001 controller, OFV 511 sensor head) attached to a translation stage (Newport Model 421) (Michelsen et al., 1982; Elias et al., 2003). Pieces of reflective tape (approx. 1 mm2) were placed at 10mm increments on the arena floor to serve as measurement points for the LDV. The laser was positioned at the closest point possible to the spiders at the start of each interaction. The LDV signal was synchronized and recorded along with the video taping of contests (Sony DVCAM DSR-20 digital VCR, 44.1 kHz audio sampling rate).
An Eppendorf tube containing an empty female nest was placed at the center of the arena. Initially, a removable opaque barrier was also placed in the arena to divide it into two equal parts, and a single male was introduced into each side of the arena. After a five minute acclimatization period, the barrier was removed. The addition of the barrier ensured that each male had a period to acclimatize and removed any resident or ownership affects from the interaction. Contest observation was terminated after three bouts were completed (see below). Most males were only used once (N=108), except for four males that were used twice (against different opponents). A minimum of seven days elapsed between the contests of males used more than once. Males were paired randomly with contestants (male weight range: 17.30– 69.90 mg; mean ± SE, N= 108 males; mean absolute size difference ± SD: 9.59 ± 7.40 mg, N=56 pairings).
After experiments were concluded, we weighed (Ohaus electronic balance) and digitally photographed (Nikon Digital Camera DXM 1200) all males using a Zeiss microscope (Stemi 2000C). We then measured two metrics for body size from the digital photographs using ACT-1 measurement software: cephalothorax (prosoma) width and patella-tibia length (an average of both front legs). As cephalothorax width was correlated strongly with male patella-tibia length (r2=0.52, p<0.0001, N = 112), we only used cephalothorax width as a measurement of male size in our analyses.
Male Behaviour
Males perform a series of stereotyped behaviours during aggressive interactions. Broadly, these behaviors can be divided into two phases: (1) a pre-contact phase prior to male-male contact, and (2) a contact phase where males physically contact each other. The pre-contact phase begins when the two spiders orient towards one another and adopt a hunched posture with their body raised above the substrate, the front pair of legs curled in front of the body and their abdomen curled underneath their body. Males then approach or retreat from one another with their front legs outstretched horizontally. During these displays, males produce a series of substrate-borne vibrations (see below). These substrate-borne signals usually precede movements towards rivals and rarely precede retreats. The contact phase begins when the two spiders are close to each other and begin to “leg fence” ("embracing" in Pollard et al., 1987). Leg fencing behaviour occurs as males attempt to push each other backwards with their front legs. A subset of these interactions escalated further to “grappling”. Grappling behaviour occured when the two males lock legs and chelicerae (jaws). A male was considered to have won a bout when the rival male turned away and retreated for more than two body lengths. Often fights occurred at the wall of the arena where losing males were not able to escape readily. In this case, winners were assessed when losers turned away and continually tried to climb the petroleum jelly coated wall for more than 2 seconds.
In all the interactions, the duration of pre-contact and contact phases were measured using Observer event recorder software (Observer Video PRO 5.0, Noldus Information Technologies). Pre-contact phases were measured from the time both males oriented towards one another to the initiation of body contact. Contact phases were measured from the initiation of body contact to the time the losing male turned away from the winning male. In addition, we recorded the number of substrate-borne signals produced by each male. Males produced signals using abdominal tremulations (Elias & Mason, data not shown) similar to other jumping spiders (Elias et al., 2003). These tremulations are visible to the naked eye and are evident in the videotaped recordings (Elias personal observation). In addition, we noted the occurrence of vibratory signals from each male during the recording procedure. All measurements were recorded for each contest bout.
Statistical analyses
In order to measure the properties of substrate-borne signals, we acquired a subset of signals (N=112) from videotapes using Adobe Premiere Pro 2.0. The temporal and spectral properties of signals were measured using Matlab (The Mathworks).
We tested for relationships in the first bout between substrate-borne signals and different male measurements using simple and stepwise backward multiple regression models. For the analysis of contest duration for each phase, we used the framework suggested by Taylor and Elwood (2003) and Morrell et al. (2005). For the first contest bout, we investigated winner and loser traits as distinct explanatory variables in simple and backward multiple regression models with contest phase duration as the dependent variable. In addition we modified some of these analyses. Taylor and Elwood (2003) correctly noted that strict reliance on composite measures based on RHP of both contestants (e.g. the absolute RHP difference between winner and loser) fails to distinguish between alternative mechanisms underlying the (frequently observed) inverse relationship between contest duration and RHP differences. Taylor and Elwood (2003) offer some useful suggestions for analysis, including the use of winner and loser RHP as independent variables in a multiple regression combined with graphical examination of simple linear regressions of contest duration on winner RHP and loser RHP, separately. However, one drawback of their approach is that it does not allow assessment of the effect of RHP differences between contestants, independent of each contestant’s absolute RHP. Comparing slopes derived from a simple (univariate) regression of duration on RHP for winners and losers separately is susceptible to effects of correlations between winner and loser RHP that may result by chance even when contestants are paired randomly. For example, if pairs frequently consisted of contestants of very similar or dissimilar RHP, then these (positive or negative) correlations between winner and loser RHP could generate a spurious relationship between duration and RHP when considering winners and losers separately (i.e. in simple linear regressions). As contests are inherently an interaction, contest duration might be expected to depend critically on the relative properties of contestants. In our analyses, we therefore included the cross-product term of (winner trait)×(loser trait) as a predictor variable in addition to winner and loser traits themselves. This provides an easily interpretable and statistically valid means to evaluate the effects of all three variables potentially influencing contest duration, without the statistical problems associated with the use of composite measures of RHP differences. Our approach has the added benefit of providing a means to quantify the difference in partial effects (i.e. standardized partial regression coefficients) of winner and loser RHP analogous to the qualitative slope differences that Taylor and Elwood (2003) suggest to be diagnostic of assessment mechanisms governing contest dynamics.
For our examination of factors leading to escalation, we excluded contests which escalated to grapples because these occurred infrequently and were distinctly different behaviours from leg fences (see below). The result of the analysis, however, was similar if grapples were included (data not shown).
In order to test which variables predict grappling behaviour and overall contest outcome in the first contest bout, we used a backward multiple logistic regression model. In order to avoid pseudo-replication, we randomly selected a focal individual from each contest using a coin flip. Only measurements on this focal individual were placed into the statistical model. In order to observe any differences between signaling behaviour between contest bouts, we used a repeated measures analysis of variance (ANOVA) procedure. In addition, we also used a backward multiple logistic regression model to test which variables predicted overall contest outcomes in subsequent contest bouts. All tests are two-tailed and summary statistics are presented as mean ± SE unless otherwise noted. We report standardized coefficients for β and adjusted r2 values. Statistical analyses were performed using SPSS 13.0 for Windows.
Results
Substrate-borne signals
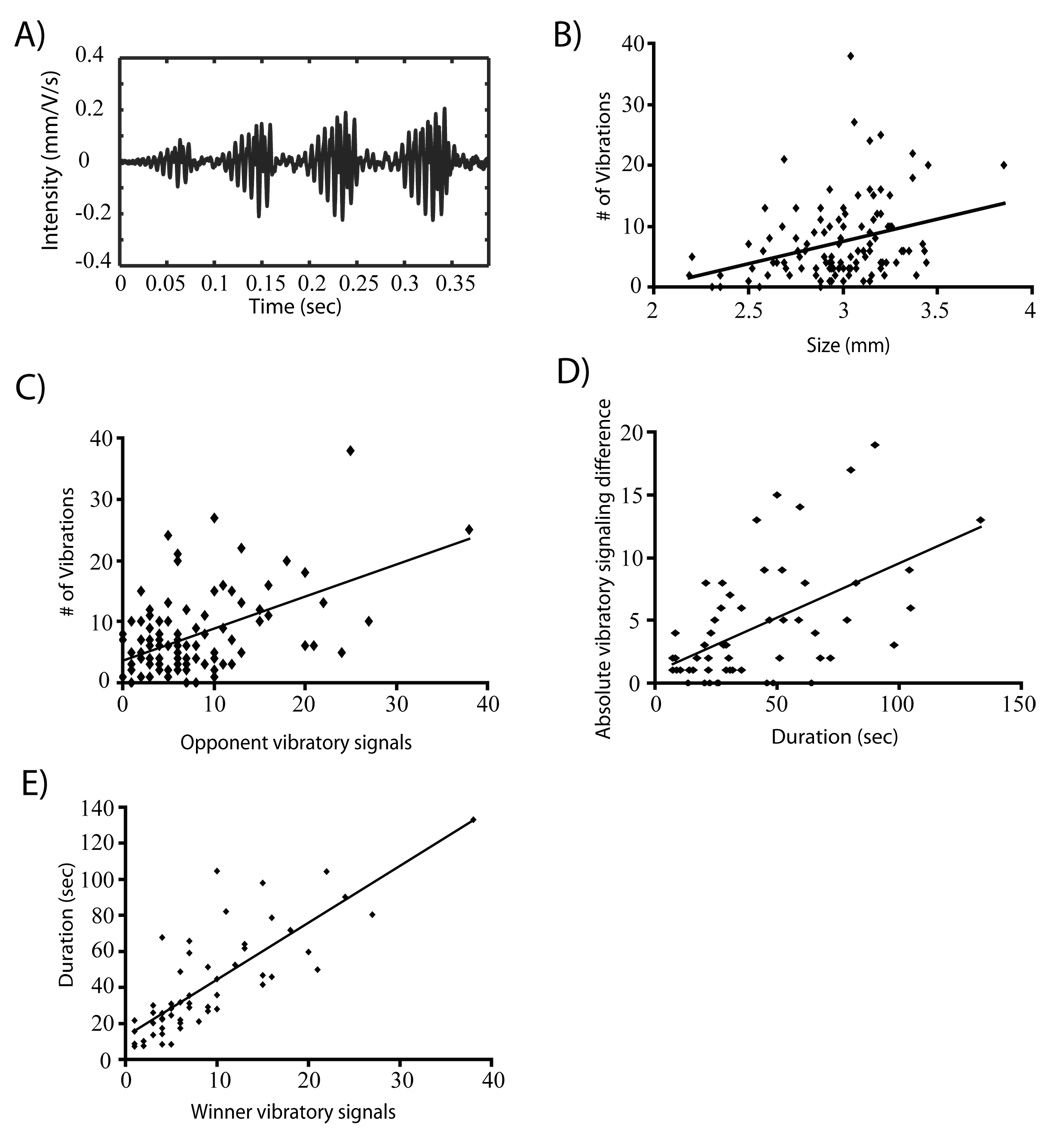
Most males produced substrate-borne vibrations. Males produce substrate-borne signals in a series of bouts consisting of 2 to 7 vibrations (Fig. 1A). Bouts occur at a frequency of 10.96 ± 0.32 Hz (N = 15) (Fig. 1A). Single vibrations are short in duration (64.69 ± 2.06 ms, N=112) and have narrow-band frequency characteristics centered at 155.4 ± 8.4 Hz (N=112). In order to determine whether any measured variables correlated with signaling, we entered weight (mg), size (cephalothorax width), and the number of opponent vibrations in a multiple stepwise regression model (Final model: r2 = 0.342, F2,108= 29.534, P<0.0001). In the final model, both the size of the signaling individual (β2=0.284, P<0.0001, Fig. 1B) and the number of vibrations by the opponent (β2=0.507, P<0.0001, Fig. 1C) significantly predicted the number of vibrations produced by an individual.
Figure 1.
Pre-contact phase in Phiddipus clarus. (A) Oscillogram of a bout of vibrational signaling. (B) The relationship between the number of vibration signals produced and size (cephalothorax width). (C) The relationship between an individual’s own vibrations and vibrations produced by opponents. (D) The relationship between vibration signaling differences and the duration of the pre-contact phase (E) The relationship between winner vibration and the duration of the pre-contact phase.
Pre-contact phase
The pre-contact phase was 41.85 ± 3.85 seconds (N=56) in duration. The absolute size difference between males significantly predicted pre-contact phase duration (r2=0.305, β=0.564, p<0.0001, N = 56, Fig. 1D). Following the procedures highlighted by Taylor and Elwood (2003) we examined the variables that predicted pre-contact phase duration. We entered weight, size, and the number of vibrations of both the winner and loser in a multiple backward stepwise regression model (Final model: r2 = 0.642, F1,54= 99.452, P<0.0001). In the final model, winner vibrations (β2=0.805, P<0.0001) significantly predicted pre-contact phase duration (Fig. 1E). In addition, when a vibration interaction term (winner vibrations×loser vibrations) was included in the multiple regression model (Final model: r2 = 0.648, F1,54= 99.453, P<0.0001) only winner vibrations (β2=0.805, P<0.0001) significantly predicted pre-contact phase duration. In simple linear regressions, both loser vibrations (r2 = 0.384, F1,54= 35.328, β=0.629, P<0.0001) and winner vibrations (r2 = 0.642, F1,54= 99.453, β=0.805, P<0.0001, Fig. 1E) predicted pre-contact phase duration. This pattern is best explained by the observation that P. clarus males vibrate as they approach opponents and do not vibrate as they retreat. Bigger males have opponents retreat more often than smaller males and bigger males are more likely to win. This pattern leads to higher numbers of vibrations for larger winning males. In addition small vibratory signaling differences (Fig. 1D) lead to shorter duration times because if males are vibrating at similar rates, they will more likely contact each other sooner as males vibrate as they approach opponents. Large differences result from one male vibrating as its opponent silently retreats.
Contact phase
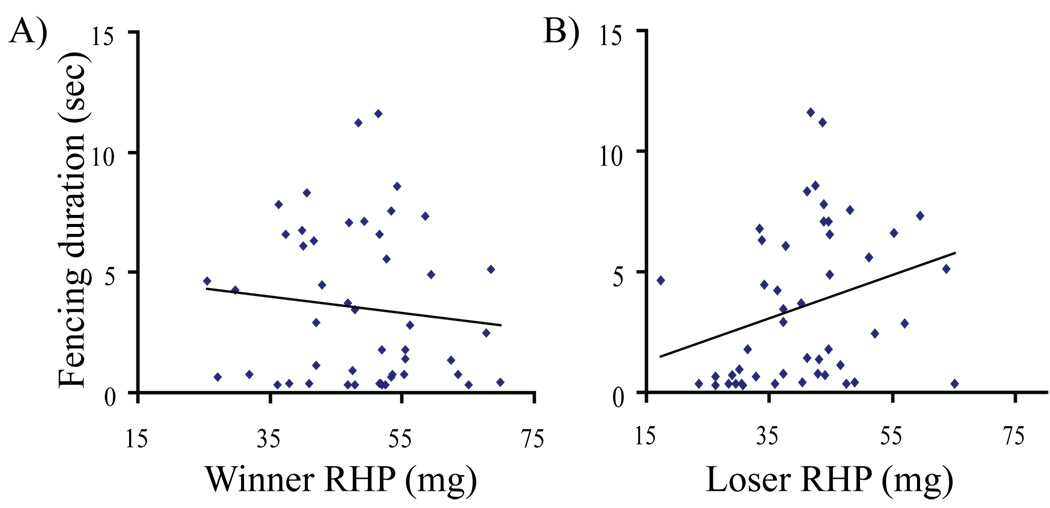
Contact phases can be divided into relatively short “fencing” escalations (3.52 ± 3.23 seconds, N =48) and relatively long “grappling” escalations (146.18 ± 91.59 seconds, N =8). The absolute weight difference between males was inversely related to contact phase duration (r2=0.213, β=−0.479, p<0.001, N = 56). Following the procedures described by Taylor and Elwood (2003) we examined the variables that predicted fencing escalation duration. We again entered weight, size, and the number of vibrations, for both winners and losers in a multiple backward stepwise regression model (Final model: r2 = 0.154, F2,45= 5.269, P<0.009). Both winner weight (β4= −0.407, P=0.017, Fig. 2A) and loser weight (β4= 0.517, P=0.003, Fig. 2B) significantly predicted contact phase duration. When a weight interaction term (winner weight×loser weight) was included in the multiple regression model (Final model: r2 = 0.166, F2,45= 5.686, P<0.006) both loser weight (β2= 1.028, P=0.002) and the interaction term (β2= −0.824, P=0.012) significantly predicted contact phase duration while winner weight did not (β1= −0.060, P=0.895). In simple linear regressions, only loser weight (r2 = 0.060, F1,46= 3.980, β=0.282, P=0.05, Fig. 2B) and not winner weight (r2 = −0.10, F1,46= 0.541, β= −0.108, P=0.466, Fig. 2A) significantly predicted contact phase duration (Fig. 2).
Figure 2.
Fencing escalations in the contact phase in Phiddipus clarus. The relationship between fencing duration and (A) winner RHP (weight) and (B) loser RHP (weight).
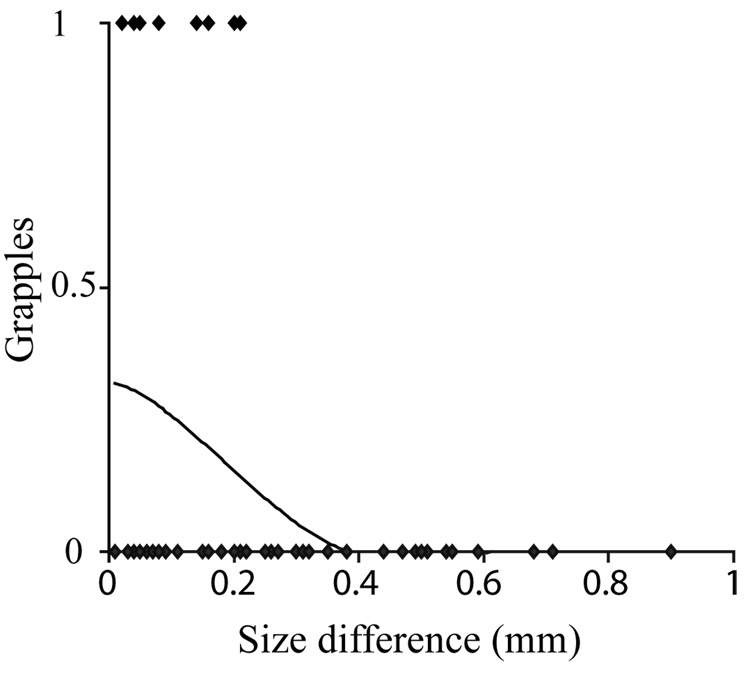
Using backward multiple logistic regression, we looked at the conditions that predicted whether or not fencing escalated to grappling. We entered absolute difference in weight, size, and vibration signaling into the model. In the final model, only size difference (β3 = −8.765, p < 0.0001, Nagelkerke R2 = 0.218) significantly predicted the occurrence of grappling with similar-sized males more likely to engage in grapples (Fig. 3). We also performed a multiple backward stepwise regression to see if any variables predicted grappling duration. The final model was not significant (Final model: r2 = 0.355, F1,6= 4.857, P = 0.070) but there was a trend for the duration of grappling to be predominantly driven by loser weight (β6=0.669, P=0.070) and not winner weight (β6=0.435, P=0.640). The final model had low power brought about by the small sample of grapples (N = 8).
Figure 3.
Grappling escalations in the contact phase Phiddipus clarus. Logistic function of the likelihood of grapple escalation and differences in overall size (cephalothorax width).
Contest outcome
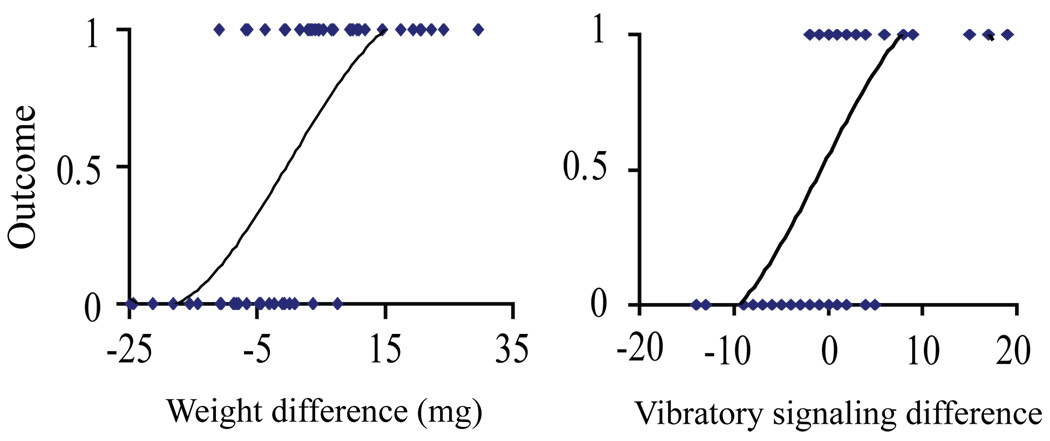
In the majority of contests (53 of 56), males that won the first bout won all three bouts. Using a backward multiple logistic regression for the first bout, we looked at the conditions that predicted whether or not individuals won contests. We entered weight, size, and vibration signaling differences into the model. In the final model (p < 0.0001, Nagelkerke R2 = 0.734), both weight difference (B2 = 0.241, p = 0.003) and vibration signaling differences (B2 = 0.350, p = 0.008) significantly predicted contest outcome with heavier and more actively signaling males winning more contests (Fig. 4).
Figure 4.
Outcome of Phiddipus clarus contests. Logistic function of the likelihood of winning and losing as a function of (A) weight differences between rivals and (B) signaling differences between rivals.
Second and third bouts
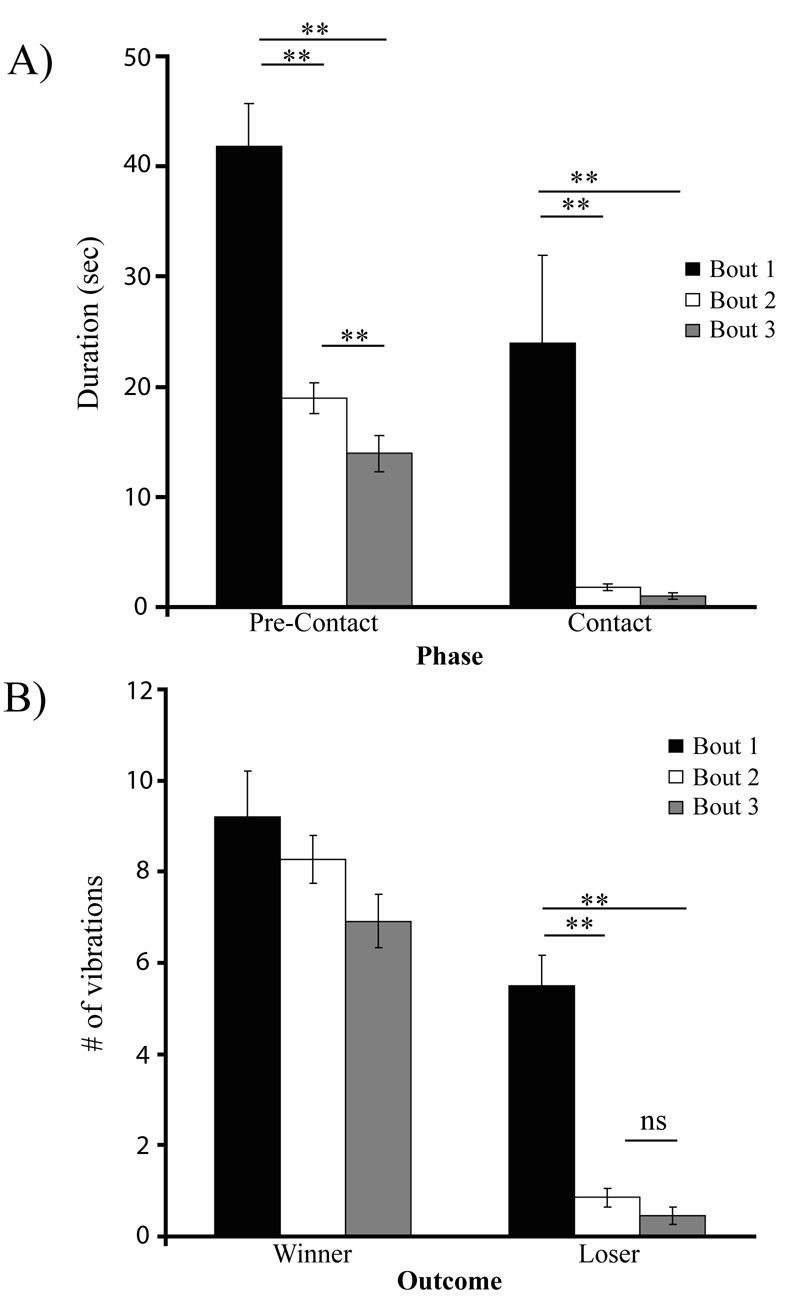
After the initial contest was concluded, we recorded detailed behaviours on two more contest bouts. In the second and third contest bouts, males spent significantly less time in pre-contact phases (repeated measures ANOVA; F1,54 = 41.218, p < 0.0001; bout 1: 41.845 ± 3.851 sec, bout 2: 18.988 ± 1.380 sec, bout 3: 13.946 ± 1.665 sec). There were significant differences in pre-contact duration between bout 1 and bout 2 (Tukey post-hoc; p<0.0001), between bout 1 and bout 3 (Tukey post-hoc; p<0.0001) and between bouts 2 and 3 (Tukey post-hoc; p = 0.02) (Fig. 5a). In the second and third contest bouts, males also spent significantly less time in contact phases (repeated measures ANOVA; F1,54 = 8.083, p < 0.001; bout 1: 23.913 ± 8.033 sec (with grapples included, N = 56), 3.535 ± 0.466 sec (without grapples N = 48); bout 2: 1.765 ± 0.315, N = 56; bout 3: 1.020 ± 0.315 sec, N = 56). Whether or not escalation included grapples, there were differences between bout 1 and bout 2 (Tukey post-hoc; p<0.001), between bout 1 and bout 3 (Tukey post-hoc; p<0.001) and between bouts 2 and 3 (Tukey post-hoc; p < 0.001). No grapples occurred in bouts 2 or 3 (Fig. 5a).
Figure 5.
Effect of experience on contests. (A) Differences between contest phase duration in different bouts. Both pre-contact and contact phase duration were significantly reduced after initial contests. (B) Difference between vibrational signaling between different contest bouts. Winners do not change signaling behaviour between bouts. Loser change their signaling behaviour between their initial (bout 1) and subsequent (bouts 2 and 3) bouts. (**p<0.001).
Vibratory behaviour also changed after the initial bout and this was dependent on contest outcome. The number of vibrations produced by the winner did not significantly change from bout to bout (repeated measures ANOVA; F1,55 = 3.611, p > 0.05; Fig. 5b). The number of vibrations produced by the loser however significantly changed between bouts (repeated measures ANOVA; F1,55 = 54.582, p < 0.0001; Fig. 5) and differences were observed between bouts 1 and 2 (Tukey post-hoc; p<0.0001), bouts 1 and 3 (p<0.0001) but not between bouts 2 and 3 (p>0.05) (Fig. 5b).
In bouts 2 and 3, contest winners were significantly predicted by differences in vibratory signaling (p < 0.0001, Nagelkerke R2 = 1.0, B3 = 10.661) and not differences in weight. Vibratory signaling differences in the model almost perfectly predicted winners in bouts 2 and 3 since losing males virtually stopped vibrating. Since vibratory signaling almost perfectly predicted contest outcome and explained all the variance in the data, weight differences were not significant in the final logistic model for bouts 2 and 3.
Discussion
Our results demonstrate that male jumping spiders use multimodal signals (visual and substrate borne) during aggressive interactions. In particular substrate-borne vibratory signals appear to be of special importance as the number of vibratory signals accurately predicted contest outcome for repeated bouts against the same opponent. Furthermore, the duration of pre-contact phases was based on differences in vibration behaviour between males. Bigger males were more willing to escalate towards contact phases even though the outcome of escalated fights was based more on weight than size. The upper limit of a male’s weight will depend on his size (larger males are heavier), but overall size is set while weight depends on the animal’s current feeding history. Thus size is ultimately an unreliable cue of fighting ability as male weight can vary greatly within size classes (Elias & Kasumovic personal observation). This may explain why males always escalated to leg fencing in the first contest bout. Once males have additional information regarding the true fighting ability (weight) of opponents, contest outcome in subsequent bouts is based on vibratory signals. Escalation in early bouts may therefore be a way to ensure honest signaling behaviour. Alternatively, the presence of a valuable resource (female nest) may explain escalation as pheromones can increase the likelihood of escalation in some jumping spider species (Cross et al., 2007).
Experience effects have been shown to be extremely important in many animal contests (reviewed in Hsu et al. 2006) and our data suggests that this is also the case for multiple contests with the same opponent in P. clarus. First, contest experience affects a male’s signaling rate; although winners signal repeatedly at the same rate, losers significantly decrease their signaling rate after losing the first bout. Second, experience appears to influence the importance of vibratory signaling behaviour in predicting contest outcome. Lastly, experience affects the time males spend in escalated fights with subsequent bouts being significantly briefer than the first bout. It is unknown how long these experience effects last and whether or not this effect would transfer to new opponents. It is possible that these experience effects could be attributed to a switch of the “rules” that govern contest outcomes. In initial contests, winners are decided by directly measuring fighting ability while in subsequent contests, males use information from multimodal signals. In the field, males may very likely escape after losing a single contest and repeated bouts with the same individual may be rare. However, our data are still valuable as they reveal that experience (particularly losing experience) can have substantial effects on subsequent behaviours. Future work will assess experience-dependent effects of P. clarus contests.
Our study suggests that contest duration, particularly when males are physically competing against each other, are based predominantly on individual thresholds (self-assessment) and to a lesser extent on opponent assessment (mutual-assessment). When loser and winner weights are considered separately, loser weight is significantly positively related to contact duration while there is a trend for winner weight to be negatively related to contact phase duration. These results match the predictions of self-assessment, since “true” mutual assessment mechanism should show an equal but opposite relationship between losers and winners (Taylor and Elwood 2003).
While supporting self assessment, our data also suggest that rival assessment may play a secondary role. In partial mutual-assessment cases, one would predict that as more rival assessment occurs, a negative relationship will develop between winner weight and contest duration (Prenter et al., 2006). Our data shows a non-significant negative trend between winner weight and contest duration, consistent with a scenario of partial mutual-assessment. More definitively, in our multiple regression model that included both winner and loser weights, both showed a significant but opposite relationship with duration, once again suggesting the contribution of mutual assessment mechanisms. When the covariance between both opponents (winner weight×loser weight) was accounted for, both loser weight and the interaction term were significant, once again suggesting a primary role for self assessment and a secondary role for mutual assessment. Mutual- and self-assessment mechanisms may thus be part of continuum of assessment strategies and males may shift between self-assessment to mutual-assessment as more information becomes available or as information becomes more reliable (Prenter et al., 2006).
Using individual thresholds (self assessment) to determine contest duration may be an economical way to accurately determine the degree of escalation, and ultimately, contest outcome while avoiding the costs associated with accurate rival assessment. The energetic demands needed to detect and process a rival’s cues and signals as well as the time needed to process the information for accurate decisions could be substantial. These costs would be even more extreme if cues and/or signals are unreliable indicators of actual fighting ability. Using individual-based thresholds to decide contests therefore allows males to pay only the costs they are willing to pay, while retaining a high likelihood of winning contests against inferior rivals. The growing number of studies demonstrating the importance of self-assessment mechanisms suggests that this mechanism could be common throughout the animal kingdom (Taylor et al., 2001; Prenter et al., 2006; Morrell et al., 2005; Bridge et al., 2000; Jennings et al., 2004; Garland & Kelly, 2006).
In a previous study, Morrell et al. (2005) observed similar results and proposed that a cumulative assessment game theory model (Payne, 1998) may explain their data. In such scenarios, no actual opponent assessment is necessary and male contest duration is based on individual thresholds (self-assessment) but a form of rival “assessment” results due to rival dependant cost accumulation (Morrell et al., 2005; Payne, 1998). Cumulative assessment games also predict that individuals of higher quality (i.e., bigger males) begin contests at higher intensity (Payne, 1998), a prediction met in P. clarus as bigger males vibrate more at the initial stages of contests. In addition, cumulative assessment games predict that as contests proceed, both contestants escalate to maintain the optimum balance between damage and energetic costs (Payne, 1998). This prediction is met in P. clarus in the ordered escalation of behaviours from multimodal displays to fencing and grappling. While cumulative assessment is a distinct possibility in P. clarus, the existence of multiple signals in aggressive displays as well as the increased importance of vibratory signals with contest experience suggest that mutual assessment mechanisms (i.e., sequential assessment games; Enquist & Leimer, 1987; Enquist & Leimer, 1983; Enquist & Leimer, 1990) also have a significant effect on contests. Our data thus suggest the possibility that contests may switch from cumulative assessment rules to sequential assessment rules as the predictive accuracy of behavioural elements increases (Enquist & Leimer, 1987; Enquist & Leimer, 1983; Enquist & Leimer, 1990; Payne, 1998; Stuart-Fox, 2006).
Our study also has implications for understanding the evolution of communication in the context of inter-male contests. In our trials, males relay information about size (both foreleg waving and vibration signals) even though size does not predict contest outcome. Thus our work shows that multiple signals can persist even if they are unreliable indicators of a male’s actual fighting ability (Bridge et al., 2000). Theoretical work has suggested that unreliable signals can evolve when costs of producing signals are relatively low and the unreliable signal offers some small Fisherian benefit (Pomiankowski & Iwasa, 1993; Pomiankowski & Iwasa, 1998; Iwasa & Pomiankowski, 1994). Our results suggest that initially unreliable signals (e.g., visual or vibratory signaling) may be ignored by the receiver, but as the information value of these signals increases due to assessment of another male cue (i.e., male fighting ability), the initially unreliable signal can become informative and processed so the receiver can make appropriate choices in subsequent decisions. Such composite effects of different signals have been hypothesized to be an important factor in the evolution of multiple signals (Johnstone & Earn, 1999; Rowe, 1999; Elias et al., 2005; Candolin & Voigt, 2003; Partan & Marler, 2005)
In summary, our results show that in the jumping spider P. clarus, even though males use multimodal signals in aggressive contests, contests are determined predominantly by a male’s assessment of his own fighting ability. However, our statistical examination of covariances between traits of rivals also suggests that mutual assessment plays a secondary role in determining contests. We conclude that the importance of mutual assessment may increase based on the reliability of information available to males as well as their previous fighting experience. Future studies are necessary to examine the role of experience in future contests against new rivals, and whether males follow the same set of rules of assessment when multiple rivals are encountered.
Acknowledgements
We would like to thank J.M. Brandt, T. Peckmezian, K. Perampaladas, S. Sivilinghem for field and laboratory assistance. We would also like to thank C. Hoefler, M. Mondanu, J. M. Brandt, and two anonymous reviewers for useful discussions and assistance. A pilot study on male signaling behaviour was completed at Cornell University with the help of S. Hatch and Dr. R.R. Hoy. We would also like to thank the Integrative Behaviour & Neuroscience Group (UTSC) for comments on the manuscript. This project was funded by a grants from National Science Foundation IRFP (0502239) and National Institute of Health NRSA (1F32GM076091-01A1) to DOE, Natural Sciences and Engineering Research Council of Canada (NSERC) PGS B, Ontario Graduate Student Fellowship, and an Animal Behaviour Society Student Grant to MMK, NSERC Discovery Grants (229029-2004 to MCBA and 238882 241419 to ACM), and grants from the Canadian Foundation for Innovation and Ontario Innovation Trust (to MCBA and ACM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bridge AP, Elwood RW, Dick JTA. Imperfect assessment and limited information preclude optimal strategies in male-male fights in the orb-weaving spider Metellina mengei. Proceedings of the Royal Society Biological Sciences Series B. 2000;267:273–279. doi: 10.1098/rspb.2000.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Elwood RW. Cumulative or sequential assessment during hermit crab shell fights: Effects of oxygen on decision rules. Proceedings of the Royal Society Biological Sciences Series B. 2000;267:2445–2452. doi: 10.1098/rspb.2000.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U, Voigt H-R. Size-dependent selection on arrival times in sticklebacks: why small males arrive first. Evolution. 2003;57:862–871. doi: 10.1111/j.0014-3820.2003.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Cross FR, Jackson RR, Pollard SD, Walker MW. Influence of optical cues from conspecific females on escalation decisions during male-male interactions of jumping spiders. Behavioural Processes. 2006;73:136–141. doi: 10.1016/j.beproc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Cross FR, Jackson RR, Pollard SD, Walker MW. Cross-modality effects during male-male interactions of jumping spiders. Behavioural Processes. 2007;75:290–296. doi: 10.1016/j.beproc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Wilkinson GS. Function of male song in the greater white-lined bat, Saccopteryx bilineata. Animal Behaviour. 2004;67:883–891. [Google Scholar]
- Draud M, Macias-Ordonez R, Verga J, Itzkowitz M. Female and male Texas cichlids (Herichthys cyanoguttatum) do not fight by the same rules. Behavioral Ecology. 2004;2004:102–108. [Google Scholar]
- Elias DO, Hebets EA, Hoy RR. Female preference for complex/novel signals in a spider. Behavioral Ecology. 2006;17:765–771. [Google Scholar]
- Elias DO, Hebets EA, Hoy RR, Mason AC. Seismic signals are crucial for male mating success in a visual specialist jumping spider (Araneae : Salticidae) Animal Behaviour. 2005;69:931–938. [Google Scholar]
- Elias DO, Mason AC, Maddison WP, Hoy RR. Seismic signals in a courting male jumping spider (Araneae : Salticidae) Journal of Experimental Biology. 2003;206:4029–4039. doi: 10.1242/jeb.00634. [DOI] [PubMed] [Google Scholar]
- Enquist M, Jakobsson S. Decision making and assessment in the fighting behavior of Nannacara anomala (Cichlidae, Pisces) Ethology. 1986;72:143–153. [Google Scholar]
- Enquist M, Leimer O. Evolution of fighting behaviour: decision rules and assessment of relative strength. Journal of Theoretical Biology. 1983;102:387–410. [Google Scholar]
- Enquist M, Leimer O. Evolution of fighting behaviour: the effect of variation in resource value. Journal of Theoretical Biology. 1987;127:187–205. [Google Scholar]
- Enquist M, Leimer O. The evolution of fatal fighting. Animal Behaviour. 1990;39:1–9. [Google Scholar]
- Faber DB, Baylis JR. Effects of body size on agonistic encounters between male jumping spiders (Araneae: Salticidae) Animal Behaviour. 1993;45:289–299. [Google Scholar]
- Gammell MP, Hardy ICW. Contest duration: sizing up the opposition? Trends in Ecology & Evolution. 2003;18:491–493. [Google Scholar]
- Garland T, Kelly SA. Phenotypic plasticity and experimental evolution. Journal of Experimental Biology. 2006;209:2344–2361. doi: 10.1242/jeb.02244. [DOI] [PubMed] [Google Scholar]
- Gwynne DT, Dadour IR. A new mechanism of sound production by courting male jumping spiders (Araneae: Salticidae, Saitis michaelseni Simon) The Zoological Society of London. 1985;207:35–42. [Google Scholar]
- Hammerstein P, Parker GA. The asymmetric war of attrition. Journal of Theoretical Biology. 1982;96:647–682. [Google Scholar]
- Hoefler CD. Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Animal Behaviour. 2007;73:943–954. [Google Scholar]
- Hsu YY, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biological Reviews. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. The evolution of mate preferences for multiple sexual ornaments. Evolution. 1994;48:853–867. doi: 10.1111/j.1558-5646.1994.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Jennings DJ, Gammell MP, Carlin CM, Hayden TJ. Effect of body weight, antler length, resource value and experience on fight duration and intensity in fallow deer. Animal Behaviour. 2004;68:213–221. [Google Scholar]
- Jennions MD, Backwell PRY. Residency and size affect fight duration and outcome in the fiddler crabUca annulipes. Biological Journal of the Linnean Society. 1996;57:293–306. [Google Scholar]
- Jensen P, Yngvesson J. Aggression between unacquainted pigs--sequential assessment and effects of familiarity and weight. Applied Animal Behaviour Science. 1998;58:49–61. [Google Scholar]
- Johnstone RA, Earn DJD. Imperfect female choice and male mating skew on leks of different sizes. Behavioral Ecology and Sociobiology. 1999;45:277–281. [Google Scholar]
- Keeley ER, Grant JWA. Visual information, resource value, and sequential assessment in Convict Cichlid (Cichlasoma nigrofasciatum) contests. Behavioral Ecology. 1993;4:345–349. [Google Scholar]
- Kokko H, López-Sepulcre A. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecology Letters. 2007 doi: 10.1111/j.1461-0248.2007.01086.x. In press. [DOI] [PubMed] [Google Scholar]
- Leimar O, Austad SN, Enquist M. A test of the sequential assessment game - fighting in the Bowl and Doily spider Frontinella pyramitela. Evolution. 1991;45:862–874. doi: 10.1111/j.1558-5646.1991.tb04355.x. [DOI] [PubMed] [Google Scholar]
- Mason AC. Territoriality and the function of song in the primitive acoustic insect Cyphoderris monstrosa (Orthoptera: Haglidae) Animal Behaviour. 1996;51:211–224. [Google Scholar]
- Maynard Smith J, Parker GA. The logic of asymmetric contests. Animal Behaviour. 1976;24:159–175. [Google Scholar]
- Mesterton-Gibbons M, Marden JH, Dugatkin LA. On wars of attrition without assessment. Journal of Theoretical Biology. 1996;181:65–83. [Google Scholar]
- Michelsen A, Fink F, Gogala M, Traue D. Plants as transmission channels for insect vibrational songs. Behavioral Ecology and Sociobiology. 1982;11:269–281. [Google Scholar]
- Morrell LJ, Backwell PRY, Metcalfe NB. Fighting in fiddler crabs Uca mjoebergi: what determines duration? Animal Behaviour. 2005;70:653–662. [Google Scholar]
- Parker GA, Rubenstein DI. Role assessment, reserve strategy, and acquisition of information in asymmetric animal conflicts. Animal Behaviour. 1981;29:221–240. [Google Scholar]
- Partan SR, Marler P. Issues in the classification of multimodal communication signals. American Naturalist. 2005;166:231–245. doi: 10.1086/431246. [DOI] [PubMed] [Google Scholar]
- Payne RJH. Gradually escalating fights and displays: The cumulative assessment model. Animal Behaviour. 1998;56:651–662. doi: 10.1006/anbe.1998.0835. [DOI] [PubMed] [Google Scholar]
- Pollard SD, Macnab AM, Jackson RR. Communication with chemicals: pheromones and spiders. In: Nentwig W, editor. Ecophysiology of Spiders. Berlin: Springer-Verlag; 1987. pp. 133–141. [Google Scholar]
- Pomiankowski A, Iwasa Y. Evolution of multiple sexual preferences by Fisher runaway process of sexual selection. Proceedings of the Royal Society of London Series B-Biological Sciences. 1993;253:173–181. [Google Scholar]
- Pomiankowski A, Iwasa Y. Runaway ornament diversity caused by Fisherian sexual selection. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5106–5111. doi: 10.1073/pnas.95.9.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenter J, Elwood RW, Taylor PW. Self-assessment by males during energetically costly contests over precopula females in amphipods. Animal Behaviour. 2006;72:861–868. [Google Scholar]
- Rowe C. Receiver psychology and the evolution of multicomponent signals. Animal Behaviour. 1999;58:921–931. doi: 10.1006/anbe.1999.1242. [DOI] [PubMed] [Google Scholar]
- Stuart-Fox D. Testing game theory models: fighting ability and decision rules in chameleon contests. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1555–1561. doi: 10.1098/rspb.2006.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PW, Hasson O, Clark DL. Initiation and resolution of jumping spider contests: Roles for size, proximity, and early detection of rivals. Behavioral Ecology and Sociobiology. 2001;50:403–413. [Google Scholar]
- Wells MS. Effects of body size and resource value on fighting behaviour in a jumping spider. Animal Behaviour. 1988;36:321–326. [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Hormones and Behavior. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]