Abstract
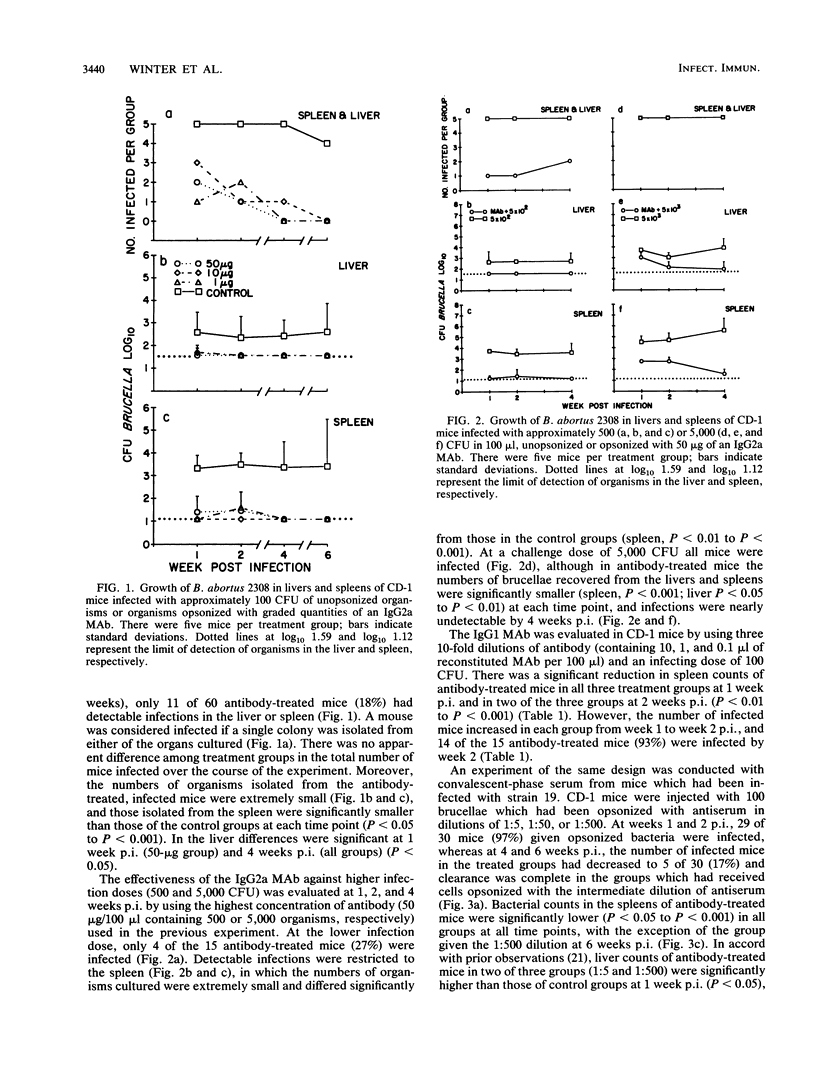
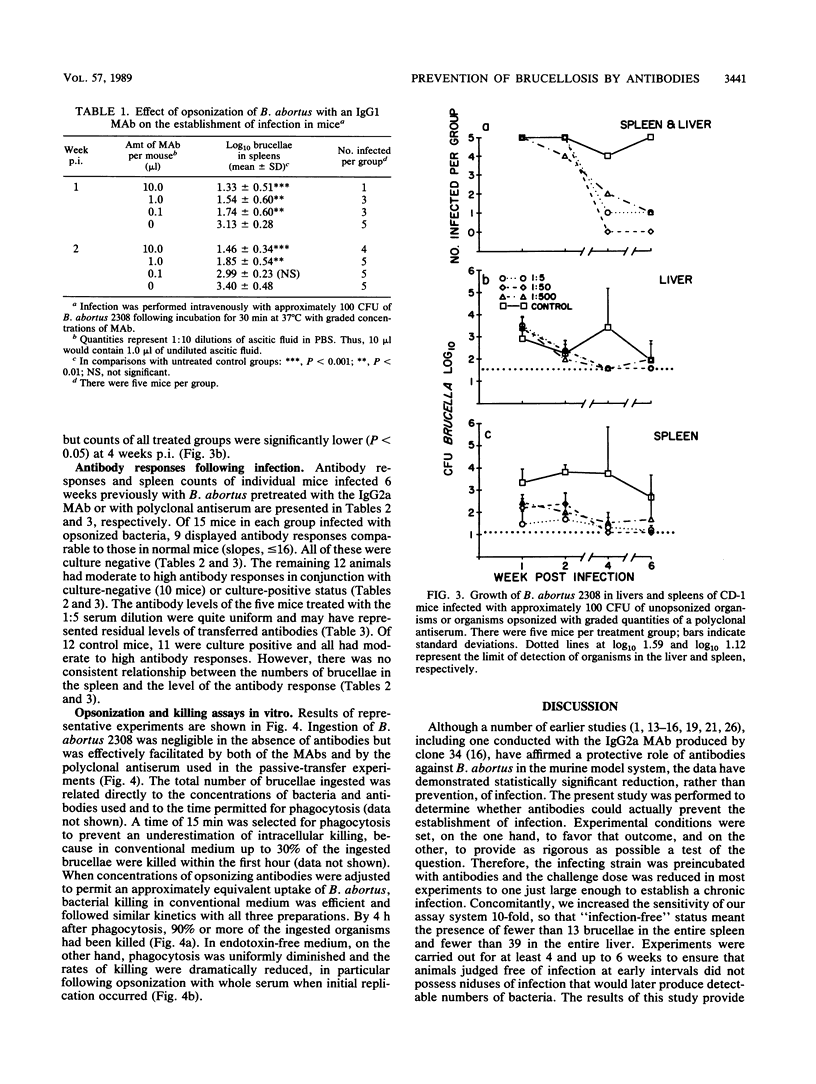
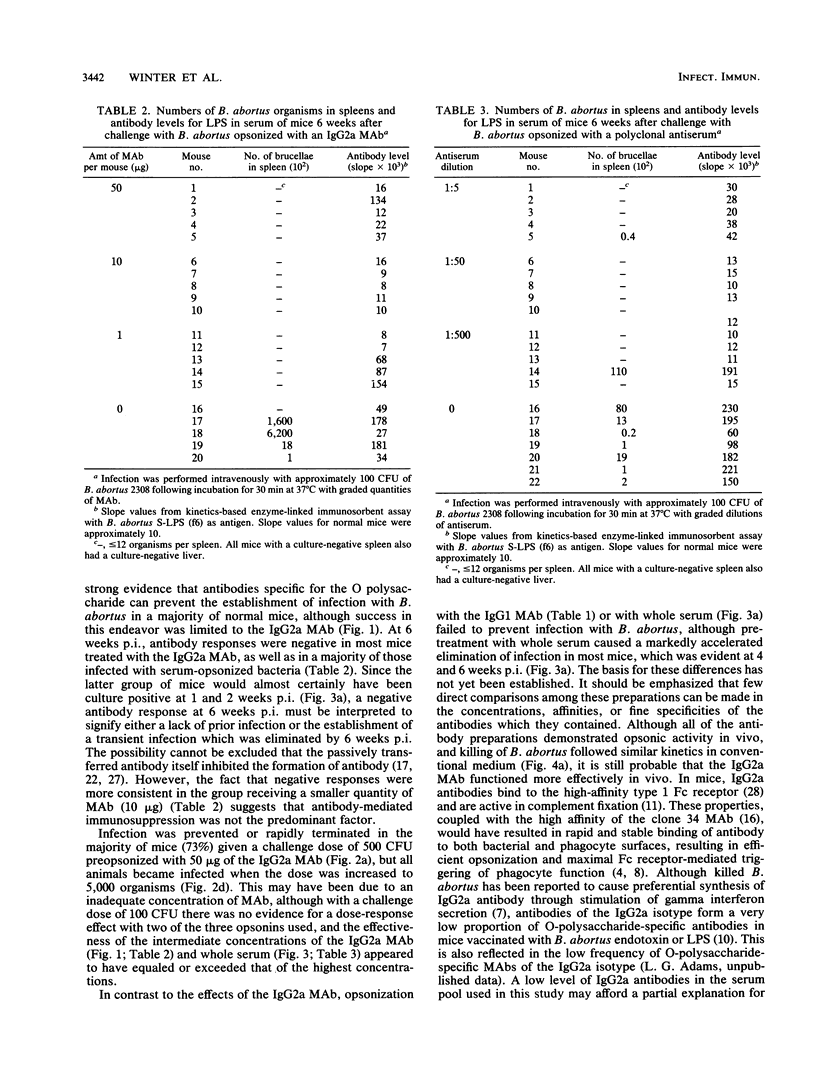
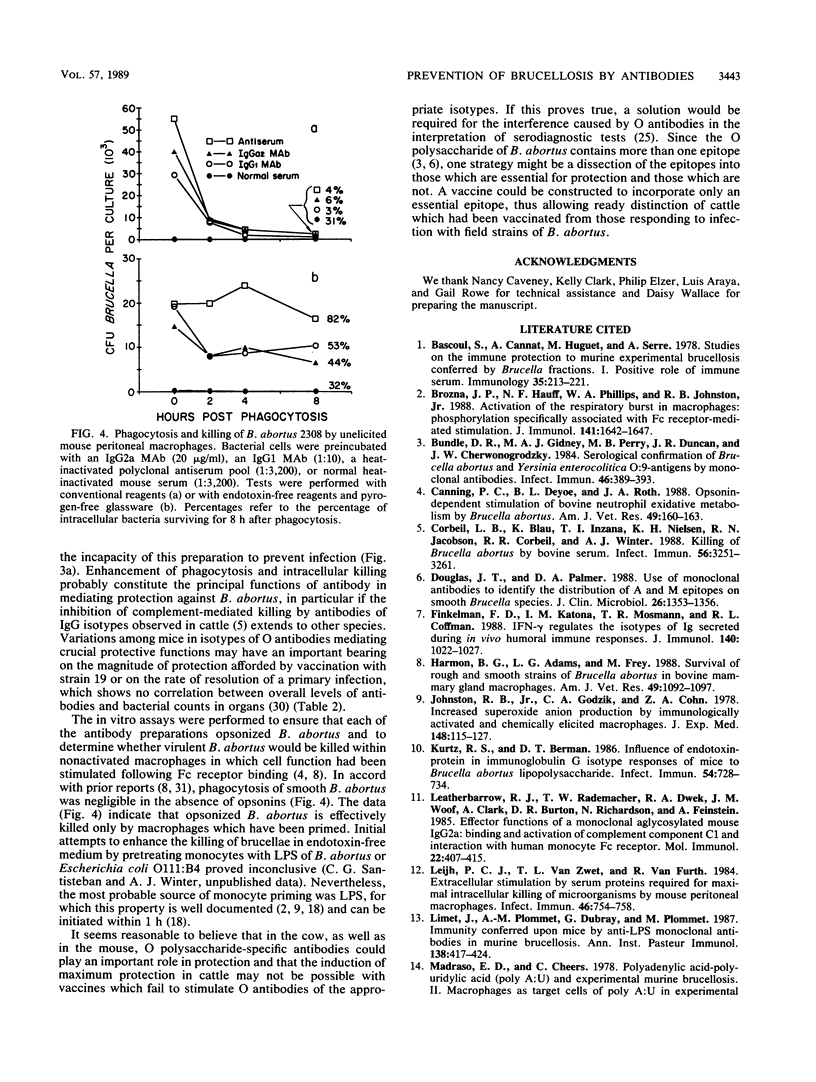
In contrast to immunity against some other facultative intracellular parasites, protective immunity against Brucella abortus is mediated in mice by antibodies as well as by cell-mediated immune responses. It was the purpose of this study to determine whether antibody alone would prevent infection with B. abortus. The majority (82%) of CD-1 outbred mice infected with 100 CFU of virulent B. abortus 2308 preincubated with graded quantities of an O polysaccharide-specific IgG2a monoclonal antibody (MAb) were free of infection 1. 2, 4, and 6 weeks later, based on detection limits of 13 brucellae per spleen and 39 per liver. Infection was present in 95% of control animals. Similar results were obtained with a challenge dose of 500 CFU, but with a challenge dose of 5,000 CFU, infection became established even with the highest concentration of MAb used (50 micrograms of MAb per 5,000 brucellae). Pretreatment with an O polysaccharide-specific IgG1 MAb or with convalescent-phase serum diminished but did not prevent establishment of infection by 100 CFU of B. abortus. A majority of culture-negative mice tested 6 weeks after infection were serologically negative, which could have signified either the absence of previous infection or the early elimination of infection. In an in vitro test system, all of the antibody preparations were efficient in opsonizing B. abortus. Effective killing of the organism by unelicited mouse peritoneal macrophages occurred in conventional but not in endotoxin-free medium, suggesting that activated macrophages were required for killing of opsonized B. abortus. These results emphasize the potential importance of antibodies in the immunoprophylaxis of brucellosis and suggest that the design of a successful vaccine will require the induction of antibodies not only of appropriate specificity but also of the optimal isotype for mediating protective functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Brozna J. P., Hauff N. F., Phillips W. A., Johnston R. B., Jr Activation of the respiratory burst in macrophages. Phosphorylation specifically associated with Fc receptor-mediated stimulation. J Immunol. 1988 Sep 1;141(5):1642–1647. [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning P. C., Deyoe B. L., Roth J. A. Opsonin-dependent stimulation of bovine neutrophil oxidative metabolism by Brucella abortus. Am J Vet Res. 1988 Feb;49(2):160–163. [PubMed] [Google Scholar]
- Corbeil L. B., Blau K., Inzana T. J., Nielsen K. H., Jacobson R. H., Corbeil R. R., Winter A. J. Killing of Brucella abortus by bovine serum. Infect Immun. 1988 Dec;56(12):3251–3261. doi: 10.1128/iai.56.12.3251-3261.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J. T., Palmer D. A. Use of monoclonal antibodies to identify the distribution of A and M epitopes on smooth Brucella species. J Clin Microbiol. 1988 Jul;26(7):1353–1356. doi: 10.1128/jcm.26.7.1353-1356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Harmon B. G., Adams L. G., Frey M. Survival of rough and smooth strains of Brucella abortus in bovine mammary gland macrophages. Am J Vet Res. 1988 Jul;49(7):1092–1097. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz R. S., Berman D. T. Influence of endotoxin-protein in immunoglobulin G isotype responses of mice to Brucella abortus lipopolysaccharide. Infect Immun. 1986 Dec;54(3):728–734. doi: 10.1128/iai.54.3.728-734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherbarrow R. J., Rademacher T. W., Dwek R. A., Woof J. M., Clark A., Burton D. R., Richardson N., Feinstein A. Effector functions of a monoclonal aglycosylated mouse IgG2a: binding and activation of complement component C1 and interaction with human monocyte Fc receptor. Mol Immunol. 1985 Apr;22(4):407–415. doi: 10.1016/0161-5890(85)90125-7. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van Zwet T. L., van Furth R. Extracellular stimulation by serum proteins required for maximal intracellular killing of microorganisms by mouse peritoneal macrophages. Infect Immun. 1984 Dec;46(3):754–758. doi: 10.1128/iai.46.3.754-758.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limet J., Plommet A. M., Dubray G., Plommet M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine brucellosis. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):417–424. doi: 10.1016/s0769-2625(87)80052-1. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986 Aug;53(2):245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Miyama-Inaba M., Masuda T., Fukuma K., Ajisaka K., Suzuki R., Kumagai K., Kanoh T., Uchino H. Inhibitory mechanism of the proliferative responses of resting B cells: feedback regulation by a lymphokine (suppressive B-cell factor) produced by Fc receptor-stimulated B cells. Immunology. 1987 May;61(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULITZEANU D. Passive protection experiments with Brucella antisera. J Hyg (Lond) 1955 Jun;53(2):133–142. doi: 10.1017/s0022172400000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher F., Lamers M. C., Dickler H. B. Antigen-antibody complexes bound to B-lymphocyte Fc gamma receptors regulate B-lymphocyte differentiation. Cell Immunol. 1985 Oct 15;95(2):368–379. doi: 10.1016/0008-8749(85)90324-7. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E. Comparative immune responses to native cell envelope antigens and the hot sodium dodecyl sulfate insoluble fraction (PG) of Brucella abortus in cattle and mice. Vet Immunol Immunopathol. 1988 Mar;18(2):149–163. doi: 10.1016/0165-2427(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Winter A. J., Rowe G. E., Duncan J. R., Eis M. J., Widom J., Ganem B., Morein B. Effectiveness of natural and synthetic complexes of porin and O polysaccharide as vaccines against Brucella abortus in mice. Infect Immun. 1988 Nov;56(11):2808–2817. doi: 10.1128/iai.56.11.2808-2817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. J., Borchert M., Kretzer F. L., Musher D. M. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J Infect Dis. 1985 Apr;151(4):682–690. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]


