Abstract
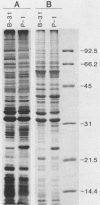
White-footed mice (Peromyscus leucopus), the primary reservoir for Borrelia burgdorferi in the northern midwest and northeastern United States, were experimentally inoculated with an infectious strain or a noninfectious strain of the Lyme disease spirochete and examined for their specific antibody response with the enzyme-linked immunosorbent assay and Western blot (immunoblot) analysis. Immunoglobulin M (IgM) anti-B. burgdorferi antibodies were detected in mice 1 to 2 days after inoculation with either the infectious or noninfectious strain of spirochetes and peaked on days 4 and 5. Mice inoculated with the infectious strain of spirochete had a secondary increase in IgM 21 days after inoculation. Mice also produced both IgG1 and IgG2 antibodies beginning 5 to 7 days after inoculation and they increased in titer until 84 days after inoculation when the experiment was terminated. Western blot analysis of sequential plasma samples from mice inoculated with the infectious strain of spirochete demonstrated the development of IgM, IgG1, and IgG2 antibodies to numerous spirochetal antigens, whereas mice inoculated with the noninfectious strain had reduced blot patterns with antibodies reactive primarily to the 31,000-kilodalton outer surface protein A. Persistent spirochetal infection in some mice, in spite of a strong and diverse antibody response, deserves further investigation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Gage K. L. Susceptibility of the black-legged tick, Ixodes scapularis, to the Lyme disease spirochete, Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):15–20. doi: 10.1016/s0176-6724(86)80096-7. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Gage K. L. Susceptibility of the hispid cotton rat (Sigmodon hispidus) to the Lyme disease spirochete (Borrelia burgdorferi). Am J Trop Med Hyg. 1987 Nov;37(3):624–628. doi: 10.4269/ajtmh.1987.37.624. [DOI] [PubMed] [Google Scholar]
- Burgess E. C., Amundson T. E., Davis J. P., Kaslow R. A., Edelman R. Experimental inoculation of Peromyscus spp. with Borrelia burgdorferi: evidence of contact transmission. Am J Trop Med Hyg. 1986 Mar;35(2):355–359. doi: 10.4269/ajtmh.1986.35.355. [DOI] [PubMed] [Google Scholar]
- Burgess E. C., Patrican L. A. Oral infection of Peromyscus maniculatus with Borrelia burgdorferi and subsequent transmission by Ixodes dammini. Am J Trop Med Hyg. 1987 Mar;36(2):402–407. doi: 10.4269/ajtmh.1987.36.402. [DOI] [PubMed] [Google Scholar]
- Coe J. E. 7S-gamma-1, 7S-gamma-2 and IgM immunoglobulins in the deer mouse (Peromyscus maniculatus). J Immunol. 1969 Oct;103(4):639–647. [PubMed] [Google Scholar]
- Coe J. E. Antigenic variation of 7Sgamma-1, 7Sgamma-2 and gamma-M globulins within the genus Peromyscus. J Immunol. 1971 Jan;106(1):34–40. [PubMed] [Google Scholar]
- Coe J. E., Coe P. R., Ross M. J. Staphylococcal protein A purification of rodent IgG1 and IgG2 with particular emphasis on syrian hamsters. Mol Immunol. 1981 Nov;18(11):1007–1012. doi: 10.1016/0161-5890(81)90119-x. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue J. G., Piesman J., Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg. 1987 Jan;36(1):92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- Godsey M. S., Jr, Amundson T. E., Burgess E. C., Schell W., Davis J. P., Kaslow R., Edelman R. Lyme disease ecology in Wisconsin: distribution and host preferences of Ixodes dammini, and prevalence of antibody to Borrelia burgdorferi in small mammals. Am J Trop Med Hyg. 1987 Jul;37(1):180–187. doi: 10.4269/ajtmh.1987.37.180. [DOI] [PubMed] [Google Scholar]
- Greene R. T., Walker R. L., Burgess E. C., Levine J. F. Heterogeneity in immunoblot patterns obtained by using four strains of Borrelia burgdorferi and sera from naturally exposed dogs. J Clin Microbiol. 1988 Nov;26(11):2287–2291. doi: 10.1128/jcm.26.11.2287-2291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi S. K., Johnson R. C. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988 Feb;56(2):314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine J. F., Wilson M. L., Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985 Mar;34(2):355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Burgdorfer W., Chappell W. A. Parasitism by Ixodes dammini (Acari: Ixodidae) and antibodies to spirochetes in mammals at Lyme disease foci in Connecticut, USA. J Med Entomol. 1984 Jan 26;21(1):52–57. doi: 10.1093/jmedent/21.1.52. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Chappell W. A. Geographic distribution of humans, raccoons, and white-footed mice with antibodies to Lyme disease spirochetes in Connecticut. Yale J Biol Med. 1984 Jul-Aug;57(4):619–626. [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Hyland K. E., Fish D., Mcaninch J. B. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J Clin Microbiol. 1988 Jun;26(6):1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Meegan J. M., Anderson J. F., Chappell W. A. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J Clin Microbiol. 1984 Aug;20(2):181–184. doi: 10.1128/jcm.20.2.181-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]