Abstract
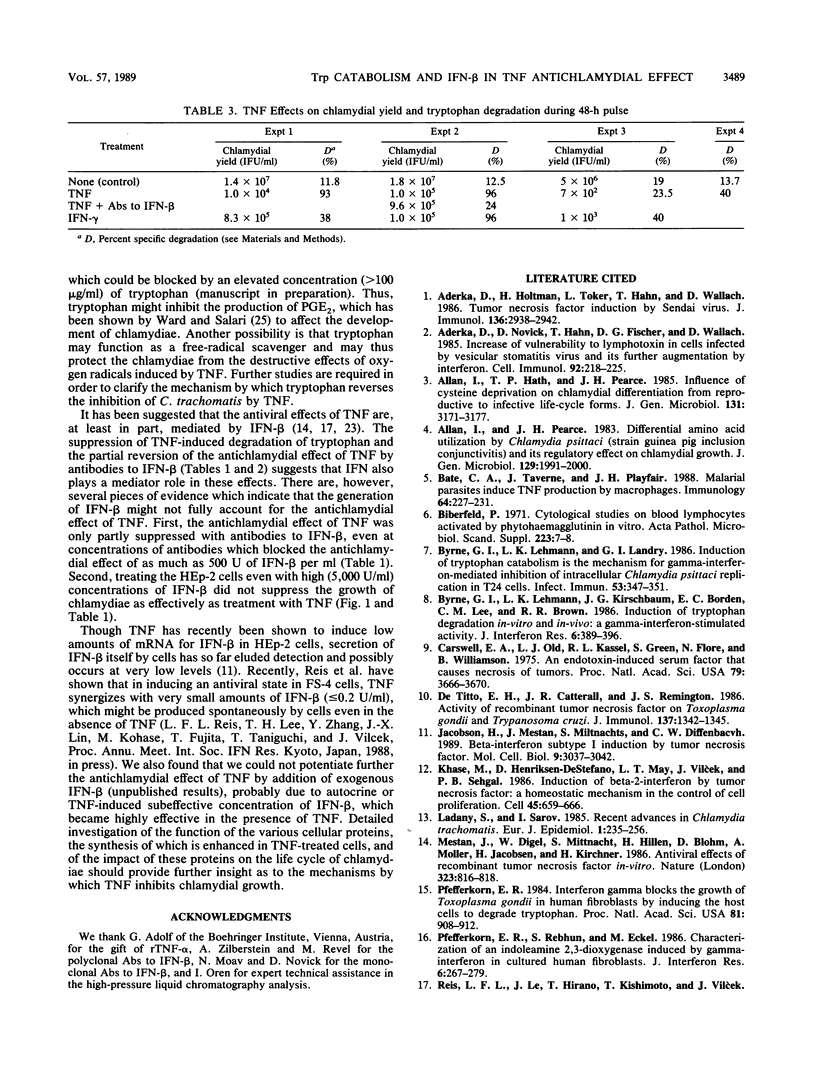
Human recombinant tumor necrosis factor-alpha (TNF-alpha) inhibited the growth of Chlamydia trachomatis (L2/434/Bu) in HEp-2 cells. The effect was synergistic with that of gamma interferon (IFN-gamma). TNF-induced resistance to chlamydiae could be blocked with cycloheximide, suggesting that it involves the function of some induced proteins. Tryptophan degradation was enhanced in the TNF-treated cells and was much further increased when the cells were treated with both TNF and IFN-gamma at concentrations at which IFN-gamma by itself had very little effect. Antibodies to IFN-beta blocked the augmentation of tryptophan degradation by TNF and decreased but did not fully eliminate the antichlamydial effect of TNF. Increased concentration of tryptophan in the growth medium (greater than 100 micrograms/ml) resulted in reversion of the antichlamydial effect of TNF. This study suggests that the inhibition of chlamydial growth by TNF is mediated partly through an autocrine function of IFN-beta which, in synergism with TNF, enhances the activity of a tryptophan-degrading enzyme(s) and partly by some other activities of TNF which can be blocked by tryptophan.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Aderka D., Novick D., Hahn T., Fischer D. G., Wallach D. Increase of vulnerability to lymphotoxin in cells infected by vesicular stomatitis virus and its further augmentation by interferon. Cell Immunol. 1985 May;92(2):218–225. doi: 10.1016/0008-8749(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Allan I., Hatch T. P., Pearce J. H. Influence of cysteine deprivation on chlamydial differentiation from reproductive to infective life-cycle forms. J Gen Microbiol. 1985 Dec;131(12):3171–3177. doi: 10.1099/00221287-131-12-3171. [DOI] [PubMed] [Google Scholar]
- Allan I., Pearce J. H. Differential amino acid utilization by Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and its regulatory effect on chlamydial growth. J Gen Microbiol. 1983 Jul;129(7):1991–2000. doi: 10.1099/00221287-129-7-1991. [DOI] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Kirschbaum J. G., Borden E. C., Lee C. M., Brown R. R. Induction of tryptophan degradation in vitro and in vivo: a gamma-interferon-stimulated activity. J Interferon Res. 1986 Aug;6(4):389–396. doi: 10.1089/jir.1986.6.389. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Lehmann L. K., Landry G. J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986 Aug;53(2):347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- Jacobsen H., Mestan J., Mittnacht S., Dieffenbach C. W. Beta interferon subtype 1 induction by tumor necrosis factor. Mol Cell Biol. 1989 Jul;9(7):3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Ladany S., Sarov I. Recent advances in Chlamydia trachomatis. Eur J Epidemiol. 1985 Dec;1(4):235–256. doi: 10.1007/BF00237099. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Rebhun S., Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res. 1986 Jun;6(3):267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- Reis L. F., Le J. M., Hirano T., Kishimoto T., Vilcek J. Antiviral action of tumor necrosis factor in human fibroblasts is not mediated by B cell stimulatory factor 2/IFN-beta 2, and is inhibited by specific antibodies to IFN-beta. J Immunol. 1988 Mar 1;140(5):1566–1570. [PubMed] [Google Scholar]
- Shemer-Avni Y., Wallach D., Sarov I. Inhibition of Chlamydia trachomatis growth by recombinant tumor necrosis factor. Infect Immun. 1988 Sep;56(9):2503–2506. doi: 10.1128/iai.56.9.2503-2506.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Van Snick J., Dinarello C. A., Billiau A. The role of interferon-beta 1 and the 26-kDa protein (interferon-beta 2) as mediators of the antiviral effect of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1867–1872. [PubMed] [Google Scholar]
- Wallach D. Cytotoxins (tumour necrosis factor, lymphotoxin and others): molecular and functional characteristics and interactions with interferons. Interferon. 1986;7:89–124. [PubMed] [Google Scholar]
- Ward M. E., Salari H. Control mechanisms governing the infectivity of Chlamydia trachomatis for hela cells: modulation by cyclic nucleotides, prostaglandins and calcium. J Gen Microbiol. 1982 Mar;128(3):639–650. doi: 10.1099/00221287-128-3-639. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Yong S., Lau S. Rapid separation of tryptophan, kynurenines, and indoles using reversed-phase high-performance liquid chromatography. J Chromatogr. 1979 Jul 13;175(2):343–346. doi: 10.1016/s0021-9673(00)89443-1. [DOI] [PubMed] [Google Scholar]