Abstract
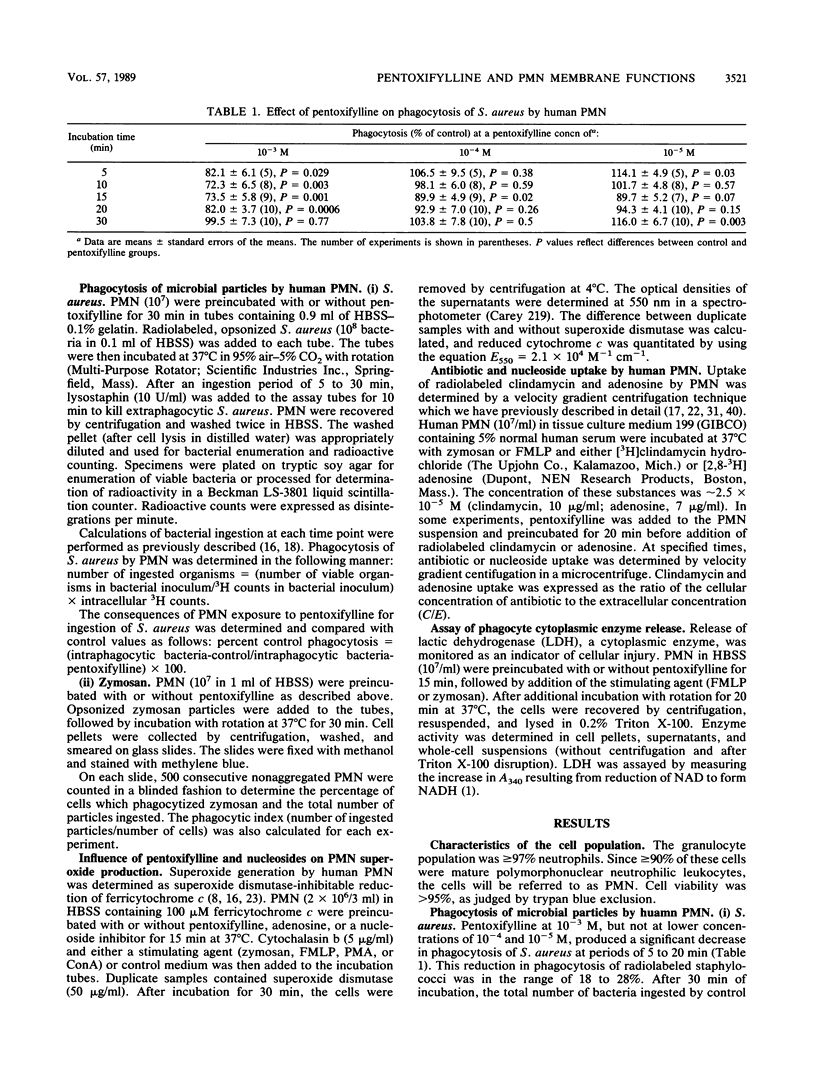
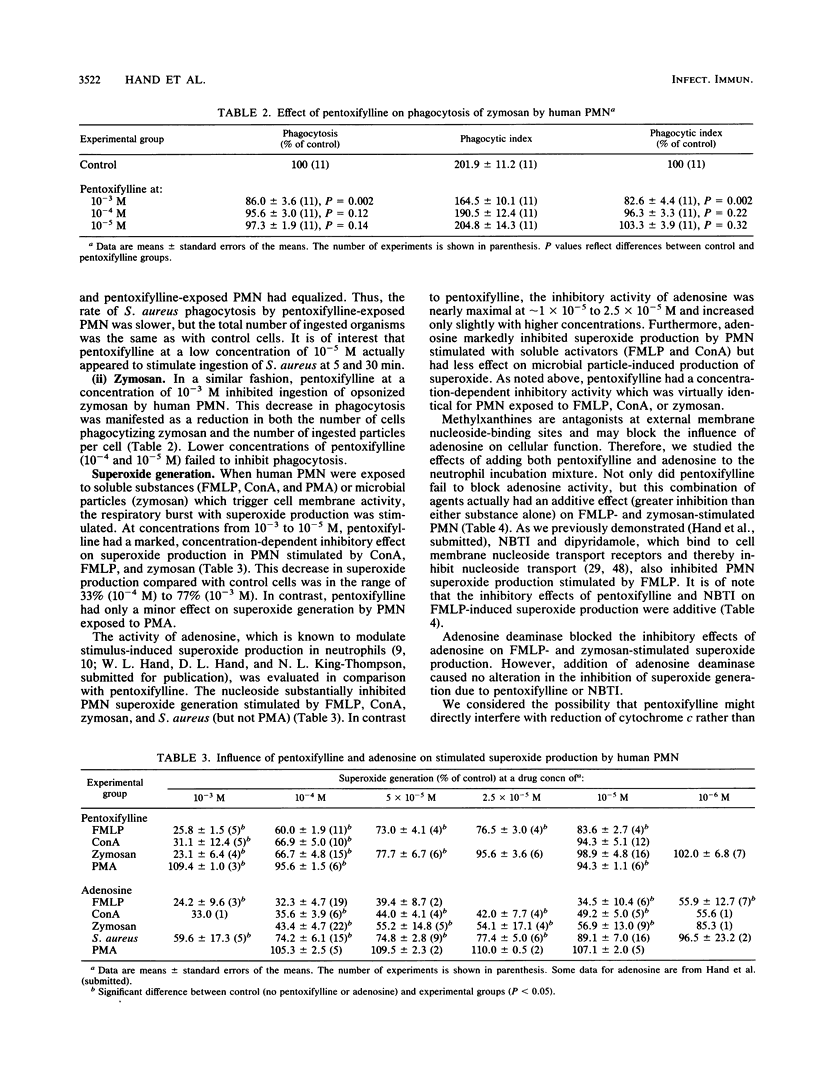
Pentoxifylline is known to have major effects on cell membrane function in mammalian cells, including human leukocytes. The protective effects of this agent in animal models of infection and inflammation may be due to alterations in phagocyte (neutrophil and macrophage) function. However, the exact mechanism of action of pentoxifylline is unknown. In this study, we evaluated the effect of the drug on several membrane-associated activities in human polymorphonuclear neutrophils and investigated possible mechanisms for the observed changes in neutrophil function. Pentoxifylline inhibited ingestion of microbial particles (Staphylococcus aureus and zymosan); decreased superoxide generation activated by zymosan, formyl-methionyl-leucyl-phenylalanine, and concanavalin A (but not phorbol myristate acetate); and decreased uptake (transport) of adenosine stimulated by formyl-methionyl-leucyl-phenylalanine and zymosan. In contrast, pentoxifylline actually increased clindamycin uptake in zymosan-stimulated polymorphonuclear neutrophils. However, pentoxifylline had no effect on uptake of adenosine or clindamycin in unstimulated neutrophils. In comparison with known inhibitors of nucleoside transport (nitrobenzylthioinosine and dipyridamole), the results suggested that pentoxifylline does not bind to membrane nucleoside transport receptors. At concentrations which inhibit neutrophil function, pentoxifylline activity is not mediated through external membrane nucleoside regulatory sites. Thus, pentoxifylline affects the activation signal chain at a point beyond the membrane receptors. Whatever its precise mechanism of action, pentoxifylline has a striking modulatory effect on cell membrane-associated responses in stimulated leukocytes and may prove useful for control of injurious inflammatory states.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Bessler H., Gilgal R., Djaldetti M., Zahavi I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 1986 Dec;40(6):747–754. doi: 10.1002/jlb.40.6.747. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptor binding: structure-activity analysis generates extremely potent xanthine antagonists. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2077–2080. doi: 10.1073/pnas.80.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chalkiadakis G. E., Kostakis A., Karayannacos P. E., Chalkiadakis M. E., Sgouromali S., Giamarellou H., Skalkeas G. D. Pentoxifylline in the treatment of experimental peritonitis in rats. Arch Surg. 1985 Oct;120(10):1141–1144. doi: 10.1001/archsurg.1985.01390340039007. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Cushley M. J., Tattersfield A. E., Holgate S. T. Adenosine antagonism as an alternative mechanism of action of methylxanthines in asthma. Agents Actions Suppl. 1983;13:109–113. [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Ehrly A. M. The effect of pentoxifylline on the deformability of erythrocytes and on the muscular oxygen pressure in patients with chronic arterial disease. J Med. 1979;10(5):331–338. [PubMed] [Google Scholar]
- Hammerschmidt D. E., Kotasek D., McCarthy T., Huh P. W., Freyburger G., Vercellotti G. M. Pentoxifylline inhibits granulocyte and platelet function, including granulocyte priming by platelet activating factor. J Lab Clin Med. 1988 Aug;112(2):254–263. [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986 Jan;29(1):135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L., Johnson J. D. Influence of bacterial-antibiotic interactions on subsequent antimicrobial activity of alveolar macrophages. J Infect Dis. 1984 Feb;149(2):271–276. doi: 10.1093/infdis/149.2.271. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Membrane transport of clindamycin in alveolar macrophages. Antimicrob Agents Chemother. 1982 Feb;21(2):241–247. doi: 10.1128/aac.21.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Newton J. A., Shigeoka A. O., Morris E., Sacchi F. Correction of a developmental defect in neutrophil activation and movement. Am J Pathol. 1987 Aug;128(2):307–314. [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Mann J. S., Cushley M. J. Adenosine as a bronchoconstrictor mediator in asthma and its antagonism by methylxanthines. J Allergy Clin Immunol. 1984 Sep;74(3 Pt 1):302–306. doi: 10.1016/0091-6749(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Ishizaka A., Wu Z. H., Stephens K. E., Harada H., Hogue R. S., O'Hanley P. T., Raffin T. A. Attenuation of acute lung injury in septic guinea pigs by pentoxifylline. Am Rev Respir Dis. 1988 Aug;138(2):376–382. doi: 10.1164/ajrccm/138.2.376. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Kristie J., Wang W. P., Eisenfeld L., Herson V. C., Maderazo E. G., Jozaki K., Kreutzer D. L. Pentoxifylline enhancement of defective neutrophil function and host defense in neonatal mice. Am J Pathol. 1987 Nov;129(2):217–222. [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. ARDS, neutrophils, and pentoxifylline. Am Rev Respir Dis. 1988 Nov;138(5):1103–1105. doi: 10.1164/ajrccm/138.5.1103. [DOI] [PubMed] [Google Scholar]
- Marcel G. A. Red cell deformability: physiological, clinical and pharmacological aspects. J Med. 1979;10(5):409–416. [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tringale S. M., Tauber A. I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986 Nov 1;137(9):2925–2929. [PubMed] [Google Scholar]
- Porter J. M., Cutler B. S., Lee B. Y., Reich T., Reichle F. A., Scogin J. T., Strandness D. E. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J. 1982 Jul;104(1):66–72. doi: 10.1016/0002-8703(82)90642-1. [DOI] [PubMed] [Google Scholar]
- Prokesch R. C., Hand W. L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982 Mar;21(3):373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose F. R., Hirschhorn R., Weissmann G., Cronstein B. N. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988 Mar 1;167(3):1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalzer E. A., Chien S. Filterability of subpopulations of leukocytes: effect of pentoxifylline. Blood. 1984 Aug;64(2):542–546. [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Wang W. P., Kreutzer D. L. Polyphosphoinositides as regulators of membrane skeletal stability. Kroc Found Ser. 1984;16:87–94. [PubMed] [Google Scholar]
- Spiegel A. M., Gierschik P., Levine M. A., Downs R. W., Jr Clinical implications of guanine nucleotide-binding proteins as receptor-effector couplers. N Engl J Med. 1985 Jan 3;312(1):26–33. doi: 10.1056/NEJM198501033120106. [DOI] [PubMed] [Google Scholar]
- Spittel J. A., Jr Pentoxifylline and intermittent claudication. Ann Intern Med. 1985 Jan;102(1):126–127. doi: 10.7326/0003-4819-102-1-126. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Hand W. L. Effects of phagocytosis on antibiotic and nucleoside uptake by human polymorphonuclear leukocytes. J Infect Dis. 1984 Mar;149(3):397–403. doi: 10.1093/infdis/149.3.397. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Ward P. A., Spengler R. N., Lynch J. P., 3rd, Larrick J., Kunkel S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1230–1236. doi: 10.1016/s0006-291x(88)81271-3. [DOI] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Novick W. J., Jr, Mandell G. L. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect Immun. 1988 Jul;56(7):1722–1729. doi: 10.1128/iai.56.7.1722-1729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Patselas T. N., Redick J. A., Mandell G. L. Enhancement of chemotaxis and protection of mice from infection. Trans Assoc Am Physicians. 1984;97:337–345. [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- Welsh C. H., Lien D., Worthen G. S., Weil J. V. Pentoxifylline decreases endotoxin-induced pulmonary neutrophil sequestration and extravascular protein accumulation in the dog. Am Rev Respir Dis. 1988 Nov;138(5):1106–1114. doi: 10.1164/ajrccm/138.5.1106. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jarvis S. M. Nucleoside transport in animal cells. Biosci Rep. 1983 Apr;3(4):309–322. doi: 10.1007/BF01122895. [DOI] [PubMed] [Google Scholar]


