Abstract
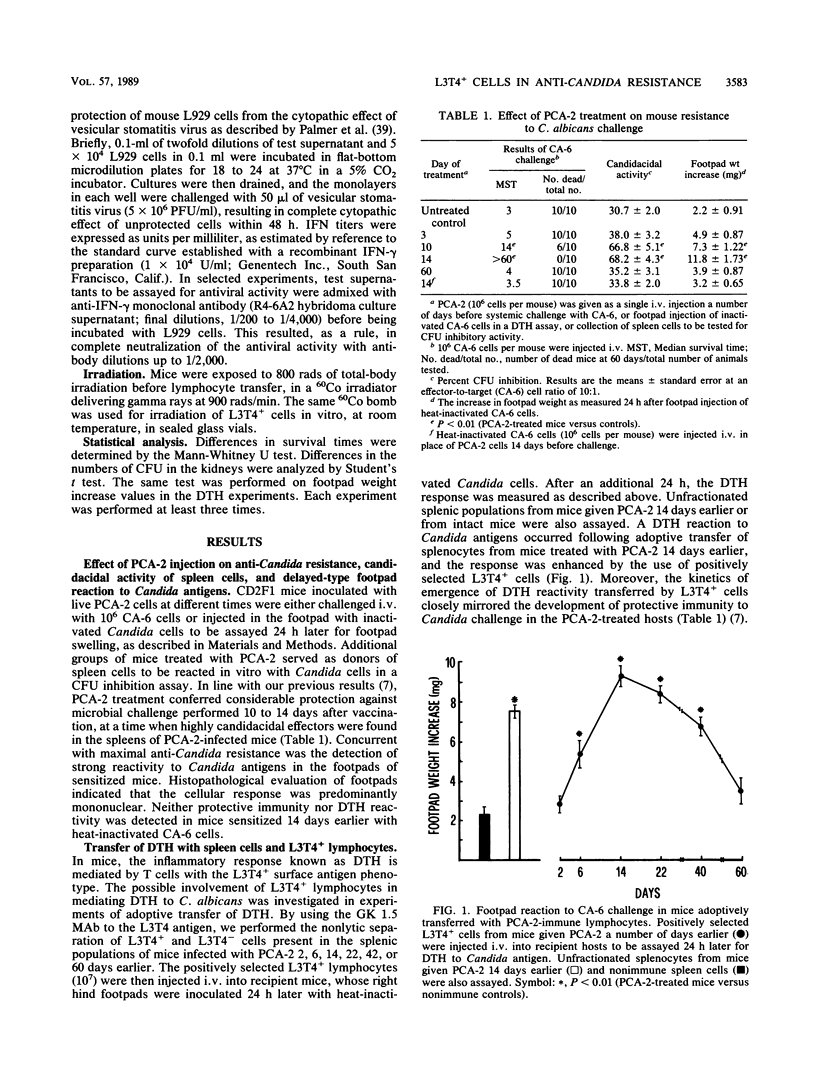
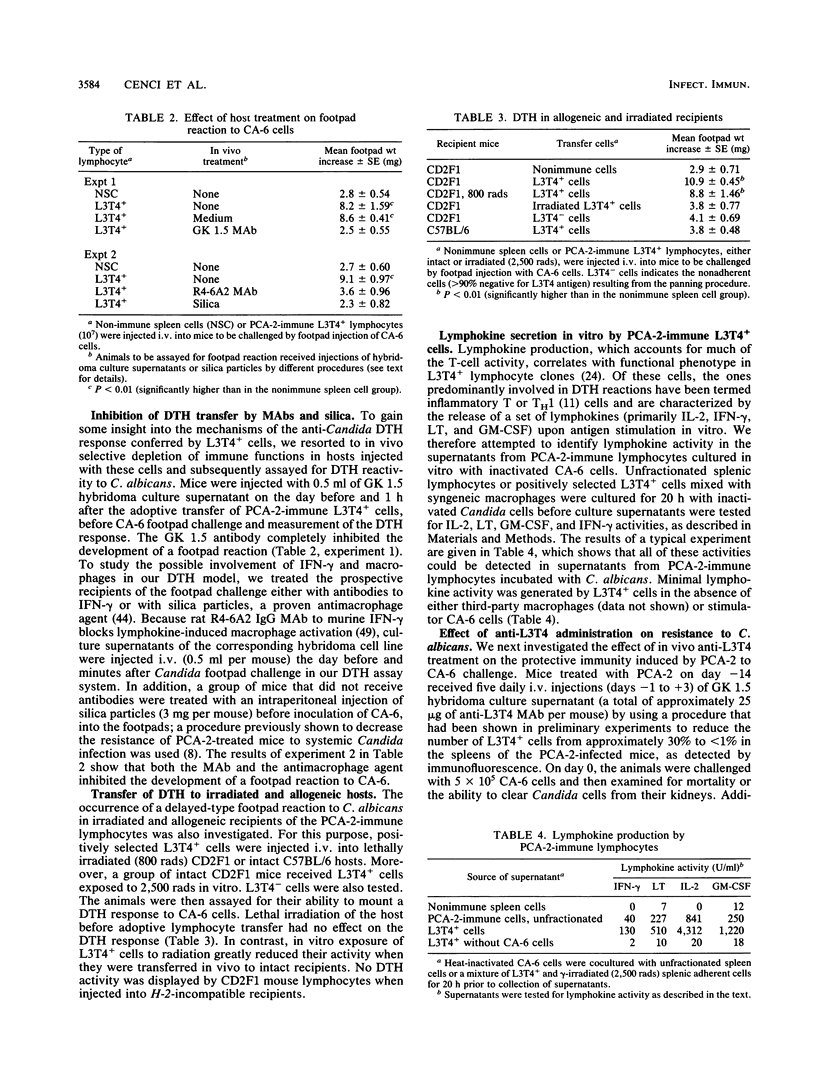
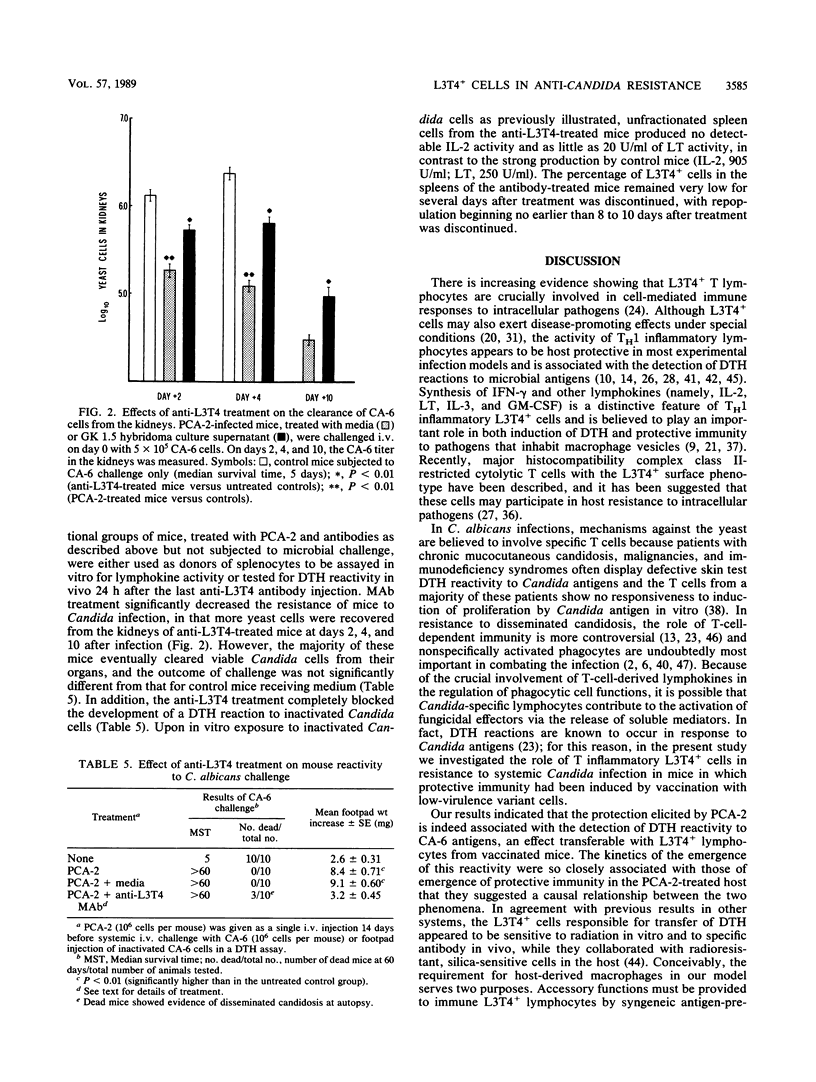
Protective immunity to lethal Candida albicans challenge in vivo and activation of splenic macrophages with highly candidacidal activity in vitro were detected in mice infected with low-virulence agerminative yeast cells of the variant strain PCA-2, at a time when a strong delayed-type hypersensitivity (DTH) reaction to C. albicans occurred in the footpads of PCA-2-treated mice. The DTH reaction was transferable with spleen cell populations from these animals, and enrichment of splenic lymphocytes in L3T4+ cells significantly increased the footpad swelling. The reactivity transferred by L3T4+ cells was a radiosensitive (2,500 rads in vitro) phenomenon that required collaboration with radioresistant, silica-sensitive syngeneic cells in the host and was inhibited by treatment of recipient mice with antibodies to the L3T4 antigen or murine gamma interferon. In vitro, the PCA-2-immune L3T4+ cells produced various lymphokine activities upon incubation with C. albicans, including gamma interferon and granulocyte-macrophage colony-stimulating factor. Anti-L3T4 monoclonal antibody treatment of PCA-2-infected mice significantly impaired their footpad reaction and resistance to C. albicans, as shown by increased recovery of yeast cells from the kidneys of anti-L3T4-treated mice. These results suggested that the mechanisms of anti-Candida resistance induced by PCA-2 may involve specific induction of a DTH response mediated by inflammatory L3T4+ T cells and lymphokine-activated phagocytic effectors. However, the survival rate of the PCA-2-immune mice challenged with C. albicans was not significantly modified by administration of the anti-L3T4 antibody, thus allowing for the conclusion that compensatory mechanisms lead to considerable anti-Candida resistance when the activity of L3T4+ cells is deficient.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Blasi E., Puccetti P., Bistoni F. Phagocytic killing of Candida albicans by different murine effector cells. Sabouraudia. 1983 Dec;21(4):271–286. [PubMed] [Google Scholar]
- Baghian A., Lee K. W. Role of activated macrophages in resistance to systemic candidosis. J Leukoc Biol. 1988 Sep;44(3):166–171. doi: 10.1002/jlb.44.3.166. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Marconi P., Puccetti P., Garaci E. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect Immun. 1983 Apr;40(1):46–55. doi: 10.1128/iai.40.1.46-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Puccetti P., Marconi P. A radiolabel release microassay for phagocytic killing of Candida albicans. J Immunol Methods. 1982 Aug 13;52(3):369–377. doi: 10.1016/0022-1759(82)90008-4. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Verducci G., Perito S., Vecchiarelli A., Puccetti P., Marconi P., Cassone A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26(5):285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Canessa A., Pistoia V., Roncella S., Merli A., Melioli G., Terragna A., Ferrarini M. An in vitro model for Toxoplasma infection in man. Interaction between CD4+ monoclonal T cells and macrophages results in killing of trophozoites. J Immunol. 1988 May 15;140(10):3580–3588. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chilgren R. A., Quie P. G., Meuwissen H. J., Hong R. Chronic mucocutaneous candidiasis, deficiency of delayed hypersensitivity, and selective local antibody defect. Lancet. 1967 Sep 30;2(7518):688–693. doi: 10.1016/s0140-6736(67)90974-9. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J. Administration of purified anti-L3T4 monoclonal antibody impairs the resistance of mice to Listeria monocytogenes infection. Infect Immun. 1989 Jan;57(1):100–109. doi: 10.1128/iai.57.1.100-109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Drysdale B. E., Zacharchuk C. M., Shin H. S. Mechanism of macrophage-mediated cytotoxicity: production of a soluble cytotoxic factor. J Immunol. 1983 Nov;131(5):2362–2367. [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. T lymphocyte-macrophage interactions in cellular antibacterial immunity. Immunobiology. 1982 Apr;161(3-4):361–368. doi: 10.1016/S0171-2985(82)80093-4. [DOI] [PubMed] [Google Scholar]
- Heidenreich S., Weyers M., Gong J. H., Sprenger H., Nain M., Gemsa D. Potentiation of lymphokine-induced macrophage activation by tumor necrosis factor-alpha. J Immunol. 1988 Mar 1;140(5):1511–1518. [PubMed] [Google Scholar]
- Hurtrel B., Langrange P. H., Michel J. C. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect Immun. 1981 Jan;31(1):95–101. doi: 10.1128/iai.31.1.95-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Carding S., Jones B., Murray J., Portoles P., Rasmussen R., Rojo J., Saizawa K., West J., Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988 Jan;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Chandler J. W., Schimke R. N. Chronic mucocutaneous moniliasis with impaired delayed hypersensitivity. Clin Exp Immunol. 1970 Mar;6(3):375–385. [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Mage M., Mathieson B., Sharrow S., McHugh L., Hämmerling U., Kanellopoulos-Langevin C., Brideau D., Jr, Thomas C. A., 3rd Preparative nonlytic separation of Lyt2+ and Lyt2- T lymphocytes, functional analyses of the separated cells and demonstration of synergy in graft-vs.-host reaction of Lyt2+ and Lyt2- cells. Eur J Immunol. 1981 Mar;11(3):228–235. doi: 10.1002/eji.1830110312. [DOI] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E. Effects of cyclophosphamide on murine candidiasis. Infect Immun. 1980 Feb;27(2):376–386. doi: 10.1128/iai.27.2.376-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E., Mather F. J. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1980 Jan;27(1):140–149. doi: 10.1128/iai.27.1.140-149.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. J. Production of gamma interferon in mice immune to Rickettsia tsutsugamushi. Infect Immun. 1984 Jan;43(1):59–65. doi: 10.1128/iai.43.1.59-65.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Pedrazzini T., Louis J. A. Functional analysis in vitro and in vivo of Mycobacterium bovis strain BCG-specific T cell clones. J Immunol. 1986 Mar 1;136(5):1828–1834. [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti P., Bianchi R., Romani L., Cenci E., Fioretti M. C. Delayed-type hypersensitivity to tumor antigens co-expressed with immunogenic determinants induced by xenogenization. Int J Cancer. 1989 Feb 15;43(2):279–284. doi: 10.1002/ijc.2910430220. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Ruthe R. C., Andersen B. R., Cunningham B. L., Epstein R. B. Efficacy of granulocyte transfusions in the control of systemic candidiasis in the leukopenic host. Blood. 1978 Sep;52(3):493–498. [PubMed] [Google Scholar]
- Schlick E., Hartung K., Chirigos M. A. Role of prostaglandin E and interferon in secretion of colony-stimulating factor by murine macrophages after in vitro treatment with biological response modifiers. Int J Immunopharmacol. 1984;6(5):407–418. doi: 10.1016/0192-0561(84)90078-x. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Mahrt J. L., Mogil R. J. Kinetics of murine delayed-type hypersensitivity response to Eimeria falciformis (Apicomplexa: Eimeriidae). Infect Immun. 1989 Jan;57(1):146–151. doi: 10.1128/iai.57.1.146-151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Holt L., Riches H. R., Hobbs J. R. Lymphocyte abnormality in chronic mucocutaneous candidiasis. Lancet. 1970 Jun 13;1(7659):1259–1261. doi: 10.1016/s0140-6736(70)91742-3. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Mazzolla R., Farinelli S., Cassone A., Bistoni F. Immunomodulation by Candida albicans: crucial role of organ colonization and chronic infection with an attenuated agerminative strain of C. albicans for establishment of anti-infectious protection. J Gen Microbiol. 1988 Sep;134(9):2583–2592. doi: 10.1099/00221287-134-9-2583. [DOI] [PubMed] [Google Scholar]


