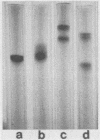
Abstract
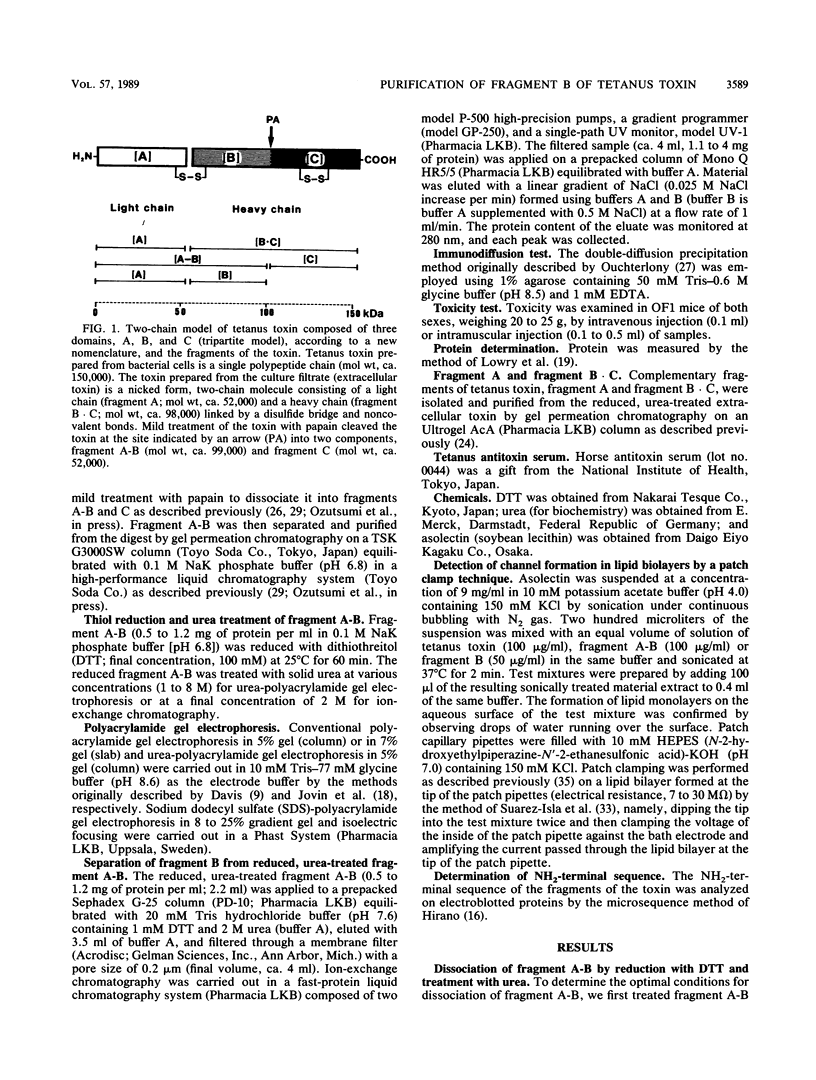
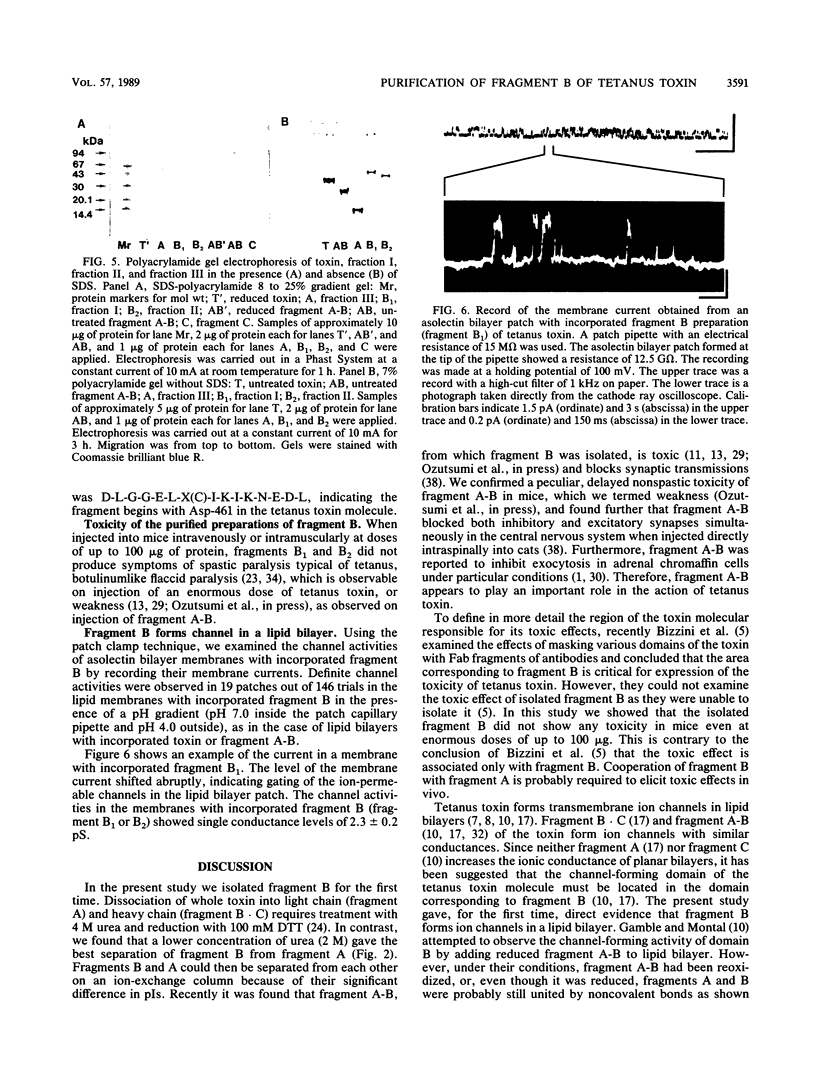
Fragment B, the N-terminal half of the heavy chain, an important domain of the tetanus neurotoxin molecule, was isolated for the first time. Tetanus toxin (composed of three domains, A, B, and C) was prepared from culture filtrates. Fragment A-B, derived from the toxin treated mildly with papain, was used for the isolation of fragment B. Fragment A-B obtained was dissociated into fragments A and B by reduction with 100 mM dithiothreitol and treatment with 2 M urea. Fragment B was separated from fragment A by ion-exchange column chromatography on a Mono Q column equilibrated with 20 mM Tris hydrochloride buffer (pH 7.6), containing 1 mM dithiothreitol and 2 M urea, in a fast-protein liquid chromatography system by elution with a linear gradient of 0 to 0.5 M NaCl. Fragment B was obtained in two forms having molecular weights of 48,000 +/- 2,000, which were indistinguishable by sodium dodecyl sulfate-gel electrophoresis or antigenic specificity, but distinguishable on polyacrylamide gel electrophoresis without sodium dodecyl sulfate and on isoelectric focusing (pI 6.7 and 7.3). The recovery of fragment B was 50 to 72% of that of fragment A-B on a molar basis. Purified fragment B was not toxic to mice on intravenous or intramuscular injection at doses of up to 100 micrograms, but was found to form channels (ca. 2.3 pS) in a lipid bilayer membrane by a patch clamp technique. The role of domain B of the tetanus toxin molecule in the mechanism of action of the toxin is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittner M. A., Holz R. W. Effects of tetanus toxin on catecholamine release from intact and digitonin-permeabilized chromaffin cells. J Neurochem. 1988 Aug;51(2):451–456. doi: 10.1111/j.1471-4159.1988.tb01059.x. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Akert K. Papain-derived fragment IIc of tetanus toxin: its binding to isolated synaptic membranes and retrograde axonal transport. Brain Res. 1981 Apr 6;210(1-2):291–299. doi: 10.1016/0006-8993(81)90902-1. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Grob P., Glicksman M. A., Akert K. Use of the B-IIb tetanus toxin derived fragment as a specific neuropharmacological transport agent. Brain Res. 1980 Jul 7;193(1):221–227. doi: 10.1016/0006-8993(80)90959-2. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Stoeckel K., Schwab M. An antigenic polypeptide fragment isolated from tetanus toxin: chemical characterization, binding to gangliosides and retrograde axonal transport in various neuron systems. J Neurochem. 1977 Mar;28(3):529–542. doi: 10.1111/j.1471-4159.1977.tb10423.x. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Toth P., Fedinec A. A. Defining a region on tetanus toxin responsible for neuromuscular blockade. Toxicon. 1988;26(3):309–318. doi: 10.1016/0041-0101(88)90222-x. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Turpin A., Raynaud M. Immunochemistry of tetanus toxin. The nitration of tyrosyl residues in tetanus toxin. Eur J Biochem. 1973 Nov 1;39(1):171–181. doi: 10.1111/j.1432-1033.1973.tb03115.x. [DOI] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov-Neori H., Yavin E., Montal M. Tetanus toxin forms channels in planar lipid bilayers containing gangliosides. Biophys J. 1984 Jan;45(1):83–85. doi: 10.1016/S0006-3495(84)84117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gambale F., Montal M. Characterization of the channel properties of tetanus toxin in planar lipid bilayers. Biophys J. 1988 May;53(5):771–783. doi: 10.1016/S0006-3495(88)83157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawade S., Bon C., Bizzini B. The use of antibody Fab fragments specifically directed to two different complementary parts of the tetanus toxin molecule for studying the mode of action of the toxin. Brain Res. 1985 May 13;334(1):139–146. doi: 10.1016/0006-8993(85)90575-x. [DOI] [PubMed] [Google Scholar]
- Habermann E., Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Ronneberger H. J., Vollerthun R., Neubauer V. Toxicity of papain-digested tetanus toxin. Pathological effect of fragment B in the absence of spastic paralysis. J Biol Chem. 1978 Jan 10;253(1):125–129. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- Hirano H. Microsequence analysis of winged bean seed proteins electroblotted from two-dimensional gel. J Protein Chem. 1989 Feb;8(1):115–130. doi: 10.1007/BF01025083. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuda M., Makinaga G., Hirai T. Studies on the antibody composition and neutralizing activity of tetanus antitoxin sera from various species of animals in relation to the antigenic substructure of the tetanus toxin molecule. Biken J. 1983 Dec;26(4):133–143. [PubMed] [Google Scholar]
- Matsuda M., Sugimoto N., Ozutsumi K., Hirai T. Acute botulinum-like intoxication by tetanus neurotoxin in mice. Biochem Biophys Res Commun. 1982 Jan 29;104(2):799–805. doi: 10.1016/0006-291x(82)90708-2. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Antigenic substructure of tetanus neurotoxin. Biochem Biophys Res Commun. 1977 Jul 11;77(1):268–274. doi: 10.1016/s0006-291x(77)80192-7. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Reconstitution of tetanus neurotoxin from two antigenically active polypeptide fragments. Biochem Biophys Res Commun. 1976 Feb 9;68(3):668–674. doi: 10.1016/0006-291x(76)91197-9. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Ozutsumi K., Sugimoto N., Matsuda M. Rapid, simplified method for production and purification of tetanus toxin. Appl Environ Microbiol. 1985 Apr;49(4):939–943. doi: 10.1128/aem.49.4.939-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Schmitt A., Dreyer F., John C. At least three sequential steps are involved in the tetanus toxin-induced block of neuromuscular transmission. Naunyn Schmiedebergs Arch Pharmacol. 1981;317(4):326–330. doi: 10.1007/BF00501314. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Hoch D. H. Neuropharmacological characterization of fragment B from tetanus toxin. J Pharmacol Exp Ther. 1985 Jan;232(1):223–227. [PubMed] [Google Scholar]
- Suarez-Isla B. A., Wan K., Lindstrom J., Montal M. Single-channel recordings from purified acetylcholine receptors reconstituted in bilayers formed at the tip of patch pipets. Biochemistry. 1983 May 10;22(10):2319–2323. doi: 10.1021/bi00279a003. [DOI] [PubMed] [Google Scholar]
- Sugimoto N., Matsuda M., Ohnuki Y., Nakayama T., Imai K. Neuromuscular blocking in acutely tetanus intoxicated mice. Biken J. 1982 Mar;25(1):21–28. [PubMed] [Google Scholar]
- Sugimoto N., Takagi M., Ozutsumi K., Harada S., Matsuda M. Enterotoxin of Clostridium perfringens type A forms ion-permeable channels in a lipid bilayer membrane. Biochem Biophys Res Commun. 1988 Oct 14;156(1):551–556. doi: 10.1016/s0006-291x(88)80877-5. [DOI] [PubMed] [Google Scholar]
- Takano K., Kirchner F., Gremmelt A., Matsuda M., Ozutsumi N., Sugimoto N. Blocking effects of tetanus toxin and its fragment [A-B] on the excitatory and inhibitory synapses of the spinal motoneurone of the cat. Toxicon. 1989;27(3):385–392. doi: 10.1016/0041-0101(89)90185-2. [DOI] [PubMed] [Google Scholar]
- Takano K. Neurophysiological aspects of tetanus toxin effects on the motor system. Eur J Epidemiol. 1985 Sep;1(3):193–201. doi: 10.1007/BF00234094. [DOI] [PubMed] [Google Scholar]