Abstract
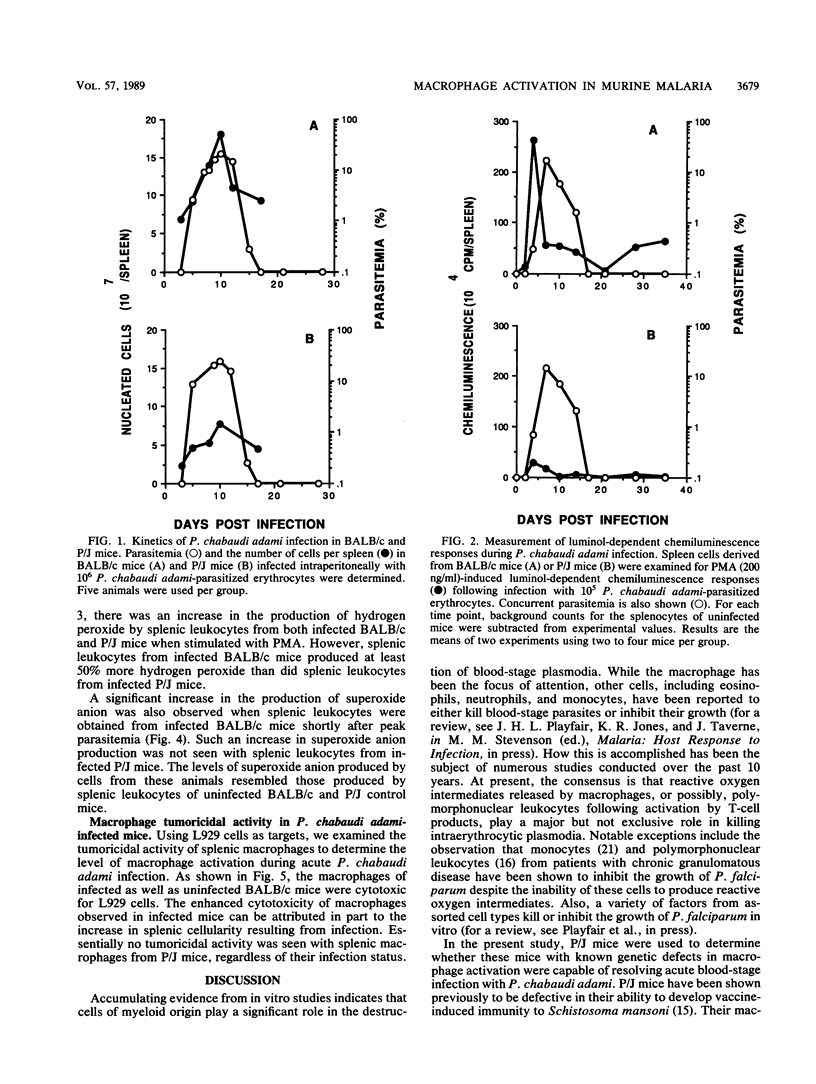
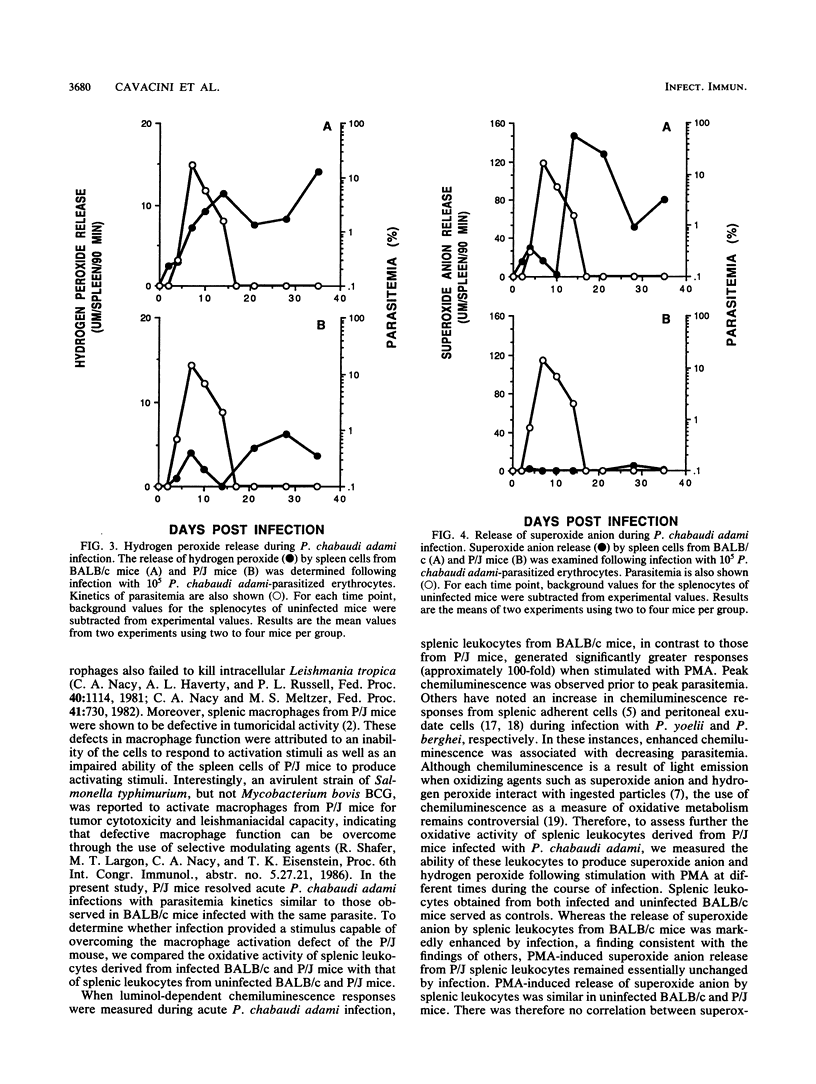
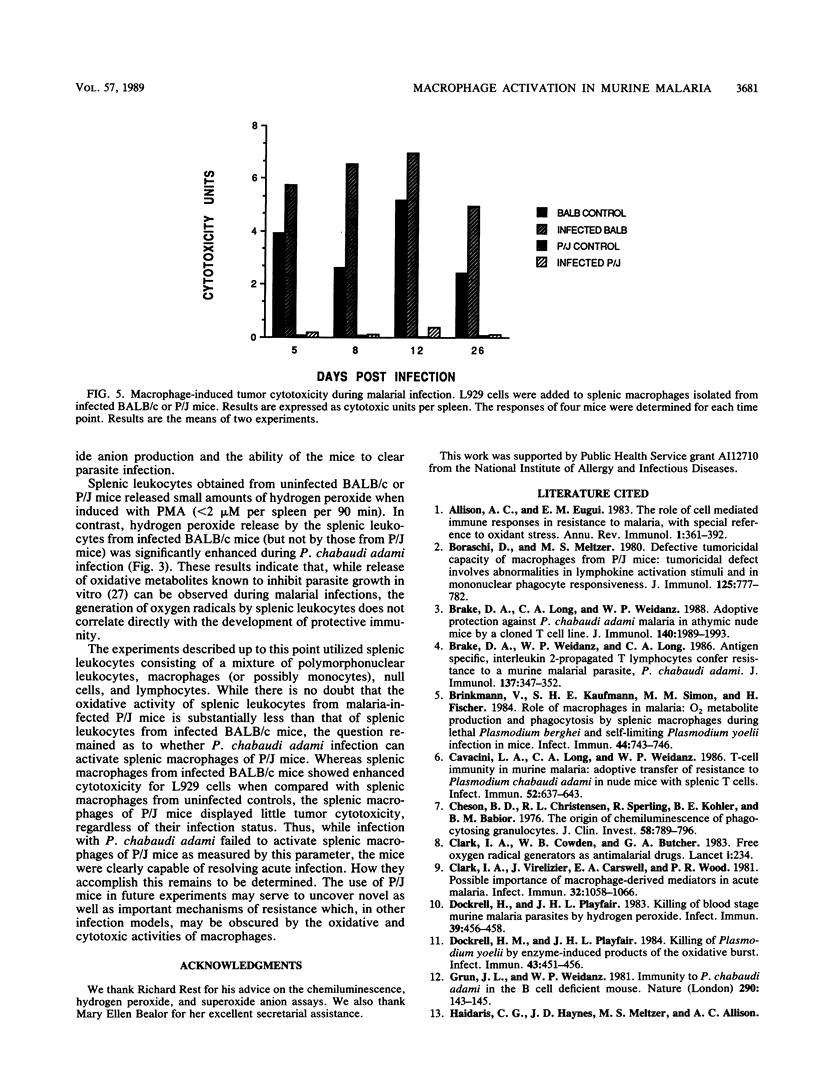
The role of splenic leukocyte oxidative activity and macrophage activation in the development of protective immunity was examined during acute Plasmodium chabaudi adami malaria. Splenic leukocyte oxidative activity was compared in infected BALB/c and P/J mice; the latter are known to suffer from defects in macrophage function. Phorbol myristate acetate-stimulated chemiluminescence and superoxide anion production by splenic leukocytes from infected BALB/c mice were found to be increased dramatically, while the response of splenic leukocytes from infected P/J mice was elevated only minimally. Hydrogen peroxide release was slightly increased in splenic leukocytes from infected BALB/c mice but remained essentially unchanged in those from infected P/J mice. Macrophage function was assessed on the basis of measurements of tumoricidal activity. Splenic macrophages from uninfected BALB/c mice displayed significant tumoricidal activity against L929 target cells. As a result of splenomegaly during infection, tumoricidal activity, when calculated on a per-spleen basis, was increased further in infected BALB/c mice. In contrast, the tumoricidal activity of splenic macrophages from P/J mice was minimal, regardless of infection. Despite these differences, both strains of mice developed malarial infections that resolved within 16 days. Thus, while the production of reactive oxygen radicals by splenic leukocytes and the phenomenon of macrophage activation have traditionally been associated with the resolution of malarial infection, this study failed to establish a correlation between these parameters and the development of protective immunity to blood-stage infection with P. chabaudi adami.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from P/J mice: tumoricidal defect involves abnormalities in lymphokine-derived activation stimuli and in mononuclear phagocyte responsiveness. J Immunol. 1980 Aug;125(2):777–782. [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brake D. A., Weidanz W. P., Long C. A. Antigen-specific, interleukin 2-propagated T lymphocytes confer resistance to a murine malarial parasite, Plasmodium chabaudi adami. J Immunol. 1986 Jul 1;137(1):347–352. [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M., Fischer H. Role of macrophages in malaria: O2 metabolite production and phagocytosis by splenic macrophages during lethal Plasmodium berghei and self-limiting Plasmodium yoelii infection in mice. Infect Immun. 1984 Jun;44(3):743–746. doi: 10.1128/iai.44.3.743-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Long C. A., Weidanz W. P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986 Jun;52(3):637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of Plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984 Feb;43(2):451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Correa-Oliveira R., Leonard E. J. Defective vaccine-induced immunity to Schistosoma mansoni in P strain mice. II. Analysis of cellular responses. J Immunol. 1984 Sep;133(3):1587–1593. [PubMed] [Google Scholar]
- Kharazmi A., Jepsen S., Valerius N. H. Polymorphonuclear leucocytes defective in oxidative metabolism inhibit in vitro growth of Plasmodium falciparum. Evidence against an oxygen-dependent mechanism. Scand J Immunol. 1984 Jul;20(1):93–96. doi: 10.1111/j.1365-3083.1984.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Li M., Li Y. J. The production of reactive oxygen species in mice infected or immunized with Plasmodium berghei. Parasite Immunol. 1987 May;9(3):293–304. doi: 10.1111/j.1365-3024.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Makimura S., Brinkmann V., Mossmann H., Fischer H. Chemiluminescence response of peritoneal macrophages to parasitized erythrocytes and lysed erythrocytes from Plasmodium berghei-infected mice. Infect Immun. 1982 Aug;37(2):800–804. doi: 10.1128/iai.37.2.800-804.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Parke L. M., Long C. A., Weidanz W. P. A method for freeing murine plasmodia of contaminating lactate dehydrogenase elevating virus. J Parasitol. 1986 Dec;72(6):956–958. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Wood P. R., Clark I. A. Macrophages from Babesia and malaria infected mice are primed for monokine release. Parasite Immunol. 1984 Jul;6(4):309–317. doi: 10.1111/j.1365-3024.1984.tb00803.x. [DOI] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


