Abstract
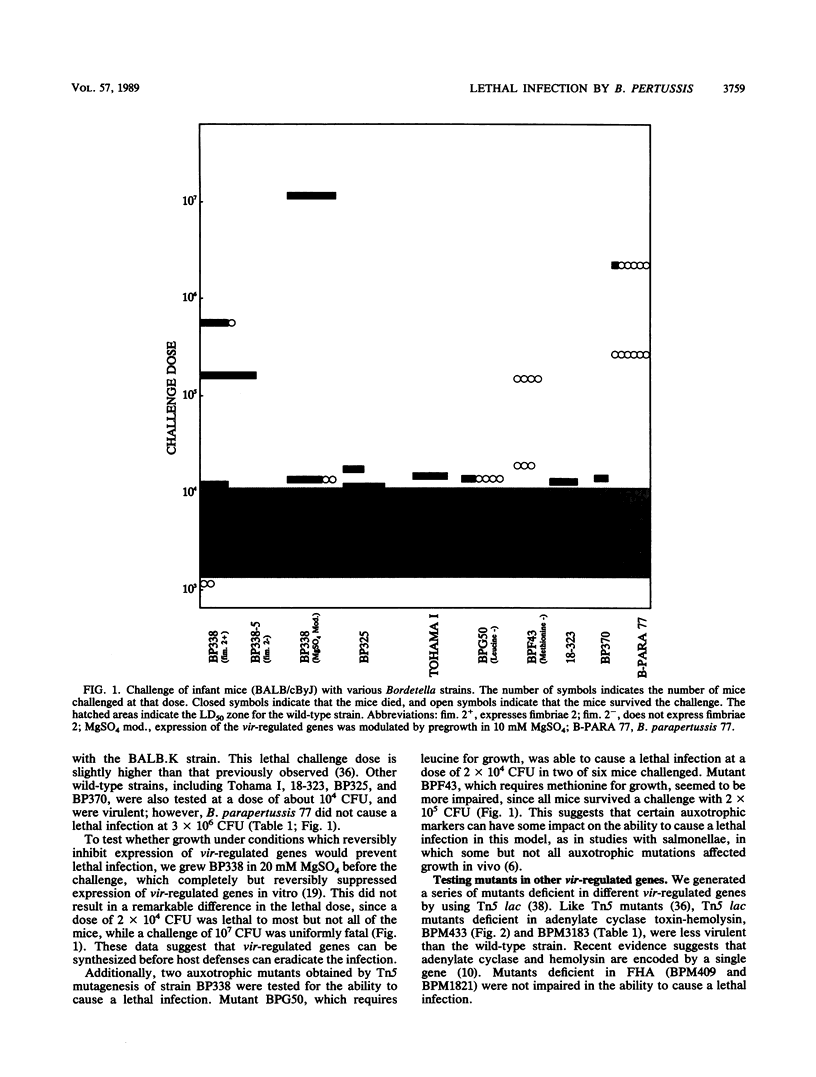
Different aspects of lethal infection of infant mice with Bordetella pertussis were examined. Mutants deficient in vir-regulated genes were tested for the ability to cause a lethal infection in the infant mouse model. Adenylate cyclase toxin-hemolysin and pertussis toxin were required to cause a lethal infection at low doses. Mixed infection caused by challenging the mice with an equal number of pertussis toxin and adenylate cyclase toxin-hemolysin mutants at a dose at which neither alone was lethal was also unable to cause a lethal infection. Production of the filamentous hemagglutinin and the dermonecrotic toxin was not required to cause a lethal infection. Nine other mutants in vir-regulated genes whose phenotypes have yet to be determined were also tested. Only two of these mutants were impaired in the ability to cause a lethal infection. Expression of fimbriae does not appear to affect the dose required to cause a lethal infection; however, fimbrial expression was correlated with the later stages of a nonlethal, persistent infection. Growth of the bacteria in MgSO4, a condition which reversibly suppresses expression of the genes required for virulence, did not alter the ability of the bacteria to cause a lethal infection. Auxotrophic mutants deficient in leucine biosynthesis were as virulent as the parental strain; however, mutants deficient in methionine biosynthesis were less virulent. A B. parapertussis strain was much less effective in promoting a lethal infection than any of the wild-type B. pertussis strains examined. A persistent infection in the lungs was observed for weeks after challenge for mice given a sublethal dose of B. pertussis, and transmission from infected infants to the mother was never observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Munoz J. J., Peacock M. G., Schad P. A., Cowell J. L., Burchall J. J., Lim M., Kent A., Steinman L., Falkow S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science. 1988 Apr 29;240(4852):656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. L., Hewlett E. L., Manclark C. R. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. doi: 10.1128/iai.25.3.896-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. F., Stocker B. A. Construction of delta aroA his delta pur strains of Salmonella typhi. J Bacteriol. 1988 Sep;170(9):3991–3995. doi: 10.1128/jb.170.9.3991-3995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Nagai M., Nakase Y. Effect of Bordetella heat-labile toxin on perfused lung preparations of guinea pigs. Microbiol Immunol. 1986;30(12):1239–1246. doi: 10.1111/j.1348-0421.1986.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Endoh M., Nagai M., Ueda T., Yoshida Y., Nakase Y. Cytopathic effect of heat-labile toxin of Bordetella parapertussis on aortic smooth muscle cells from pigs or guinea pigs. Microbiol Immunol. 1988;32(4):423–428. doi: 10.1111/j.1348-0421.1988.tb01401.x. [DOI] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin S. A., Heifetz S. A., Kasina A. Experimental respiratory infection with Bordetella pertussis in mice: comparison of two methods. Clin Invest Med. 1988 Aug;11(4):297–303. [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Brennan M. J., David J. L., Carter P. H., Cowell J. L., Manclark C. R. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect Immun. 1988 Dec;56(12):3184–3188. doi: 10.1128/iai.56.12.3184-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Cowell J. L., Brennan M. J., Burns D. L., Manclark C. R. Agglutinating monoclonal antibodies that specifically recognize lipooligosaccharide A of Bordetella pertussis. Infect Immun. 1988 Mar;56(3):699–702. doi: 10.1128/iai.56.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Barstad P. A., Coligan J. E., Mayer L., Munoz J. J., Smith S. G., Keith J. M. Molecular cloning of pertussis toxin genes. Nucleic Acids Res. 1986 Apr 25;14(8):3251–3261. doi: 10.1093/nar/14.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitto K. S., Munoz J. J., Keith J. M. Detection of subunits of pertussis toxin in Tn5-induced Bordetella mutants deficient in toxin biological activity. Infect Immun. 1987 May;55(5):1309–1313. doi: 10.1128/iai.55.5.1309-1313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Hewlett E. L., Peppler M. S., Selander R. K. Genetic diversity and relationships in populations of Bordetella spp. J Bacteriol. 1986 Apr;166(1):230–237. doi: 10.1128/jb.166.1.230-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M., Furman B. L., Wardlaw A. C. Bordetella pertussis respiratory tract infection in the mouse: pathophysiological responses. J Infect Dis. 1980 Jul;142(1):56–66. doi: 10.1093/infdis/142.1.56. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P. A., Schauer D., Orndorff P. E. Metastable regulation of type 1 piliation in Escherichia coli and isolation and characterization of a phenotypically stable mutant. J Bacteriol. 1986 Oct;168(1):179–185. doi: 10.1128/jb.168.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Robbins K., Barrera O., Corwin D., Koomey J. M. Gene conversion involving the pilin structural gene correlates with pilus+ in equilibrium with pilus- changes in Neisseria gonorrhoeae. Cell. 1986 Oct 24;47(2):267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Nedelman J., Hendley J. O., Hewlett E. L. Species specificity of Bordetella adherence to human and animal ciliated respiratory epithelial cells. Infect Immun. 1983 Nov;42(2):692–695. doi: 10.1128/iai.42.2.692-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A., Rich R., Zak F., Zak O. Filamentous hemagglutinin and pertussis toxin promote adherence of Bordetella pertussis to cilia. Dev Biol Stand. 1985;61:197–204. [PubMed] [Google Scholar]
- Urisu A., Cowell J. L., Manclark C. R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986 Jun;52(3):695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Genetic studies of the molecular basis of whooping cough. Dev Biol Stand. 1985;61:11–19. [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Melton A. R., Walker K. E., Andraos-Selim C., Meidl J. J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989 Sep;57(9):2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Franklin R., Cryz S. J., Jr, Ganss M., Peppler M., Ewanowich C. Development of a rat model for respiratory infection with Bordetella pertussis. Infect Immun. 1989 Apr;57(4):1018–1024. doi: 10.1128/iai.57.4.1018-1024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


