Abstract
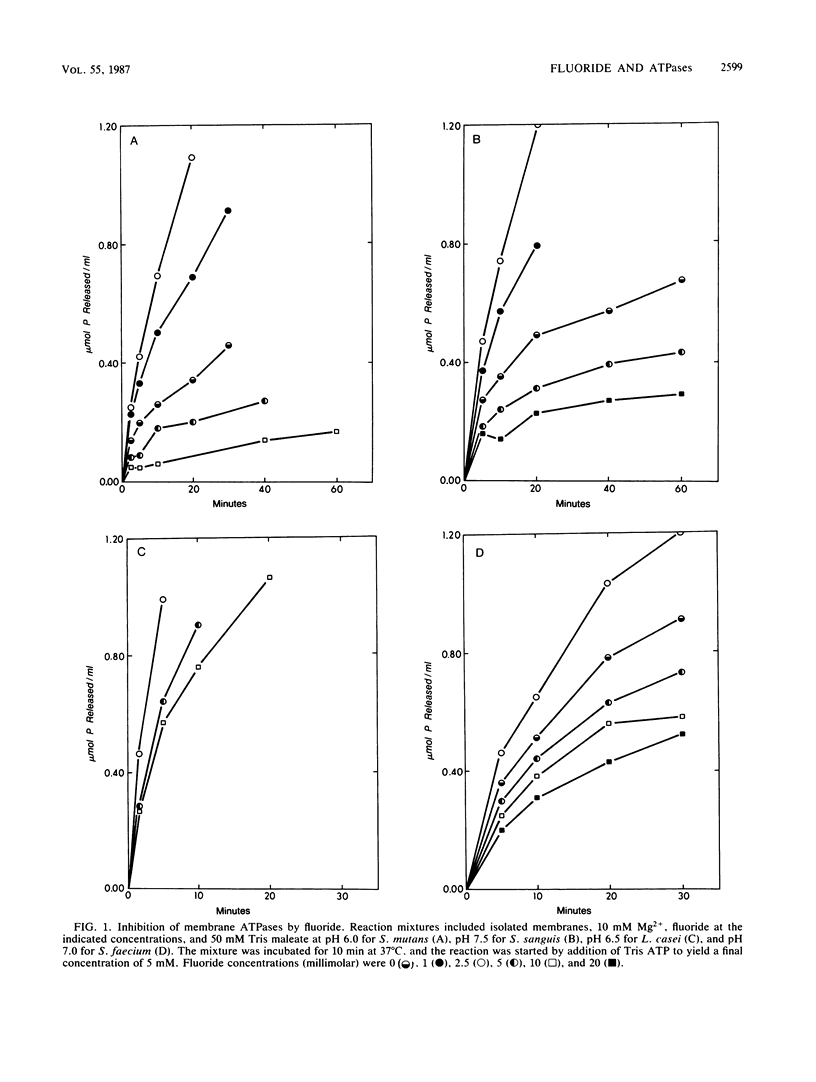
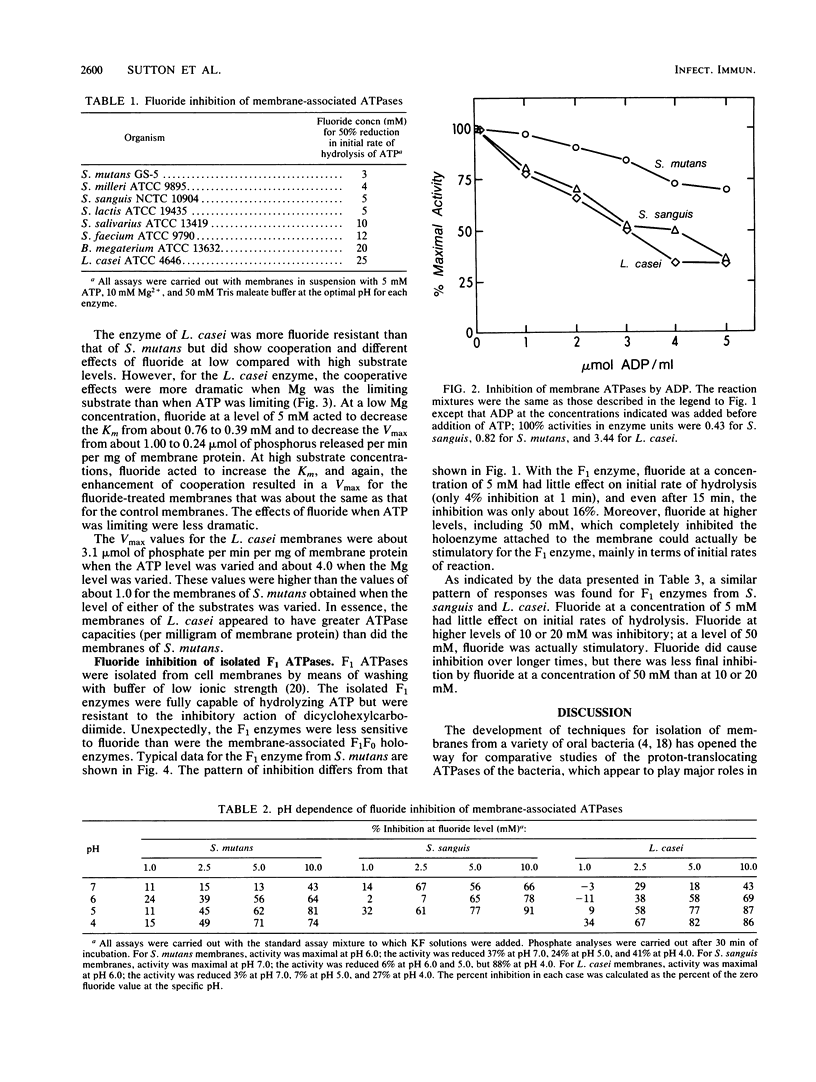
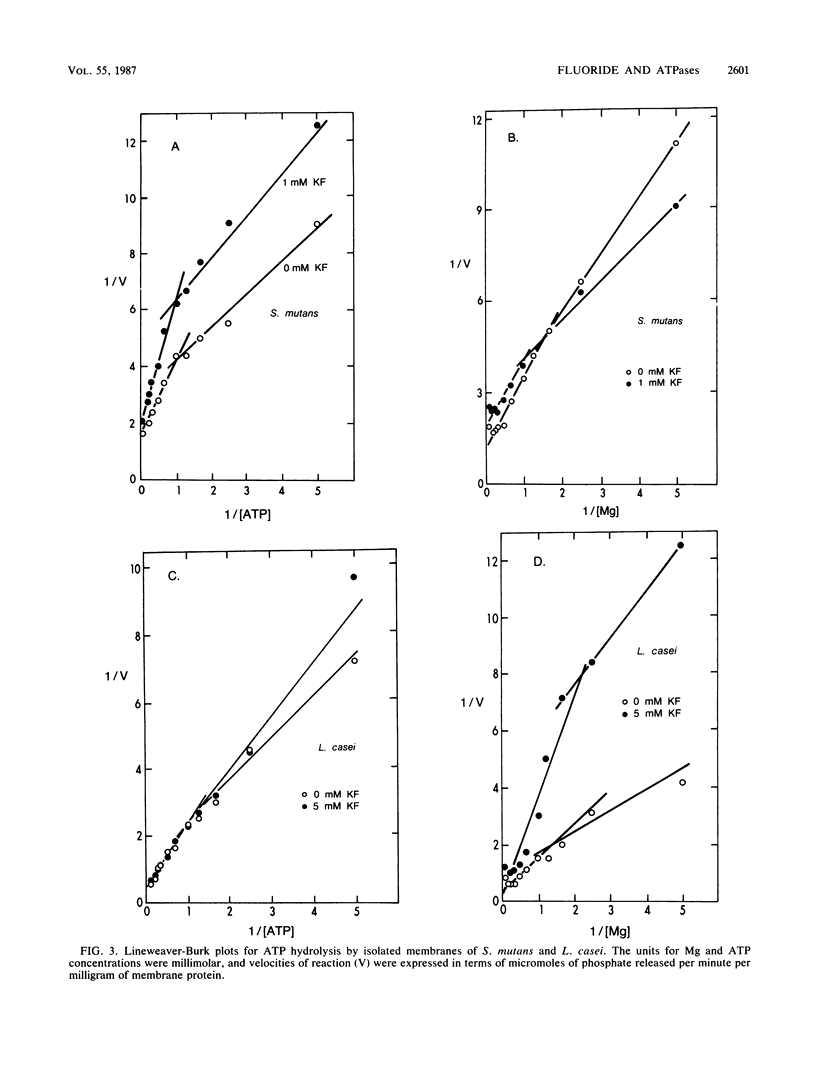
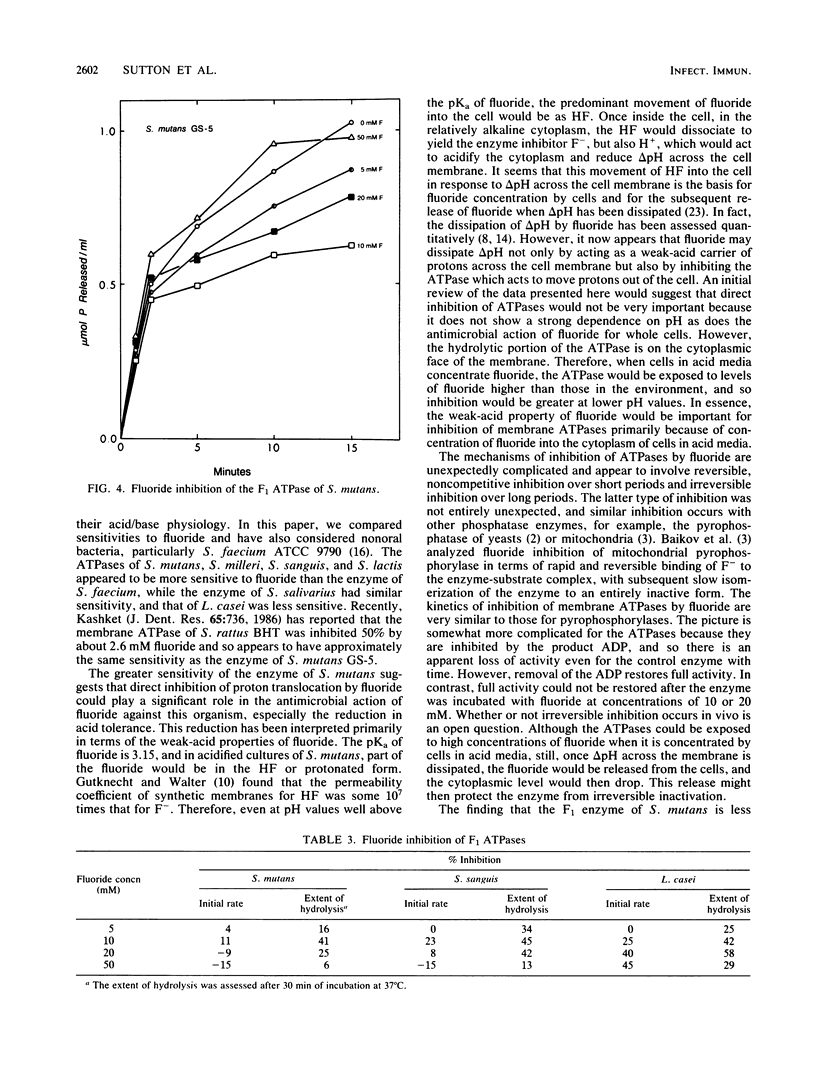
The ATPases of isolated membranes of lactic acid bacteria were found to be inhibited by fluoride in a complex manner. Among the enzymes tested, that of Streptococcus mutans GS-5 was the most sensitive to fluoride, and the initial rate of hydrolysis of ATP was reduced 50% by approximately 3 mM fluoride. The enzyme of Lactobacillus casei ATCC 4646 was the most resistant, and about 25 mM fluoride was required for 50% inhibition. The response to fluoride appeared to involve reversible, noncompetitive inhibition during short exposure to low levels of fluoride and nonreversible inhibition at higher fluoride levels. In addition, kinetic studies of the effects of fluoride on the enzymes of membranes of S. mutans and L. casei indicated that reversible inhibition was at least partly overcome at high levels of either ATP or Mg. The effects of pH on fluoride inhibition of ATPases were markedly different from the effects of pH on inhibition of acid/base regulation of intact cells by fluoride. It appeared that formation of HF was not required for inhibition of the ATPases. F1 ATPases isolated from the membranes by washing with buffers of low ionic strength proved to be less sensitive to fluoride than the membrane-associated F1F0 holoenzymes, and it was concluded that the F0 or membrane sector of the holoenzyme is involved in fluoride inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- Baykov A. A., Artjukov A. A., Avaeva S. M. Fluoride inhibition of inorganic pyrophosphatase. I. Kinetic studies in a Mg2+-PPi system using a new continuous enzyme assay. Biochim Biophys Acta. 1976 May 13;429(3):982–992. doi: 10.1016/0005-2744(76)90343-0. [DOI] [PubMed] [Google Scholar]
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner T. R., Marquis R. E. Why do bacterial protoplasts burst in hypotonic solutions? Biochim Biophys Acta. 1969;183(3):544–558. doi: 10.1016/0005-2736(69)90168-0. [DOI] [PubMed] [Google Scholar]
- DAWES C., JENKINS G. N., HARDWICK J. L., LEACH S. A. THE RELATION BETWEEN THE FLUORIDE CONCENTRATIONS IN THE DENTAL PLAQUE AND IN DRINKING WATER. Br Dent J. 1965 Aug 17;119:164–167. [PubMed] [Google Scholar]
- Eisenberg A. D., Bender G. R., Marquis R. E. Reduction in the aciduric properties of the oral bacterium Streptococcus mutans GS-5 by fluoride. Arch Oral Biol. 1980;25(2):133–135. doi: 10.1016/0003-9969(80)90089-8. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Walter A. Hydrofluoric and nitric acid transport through lipid bilayer membranes. Biochim Biophys Acta. 1981 Jun 9;644(1):153–156. doi: 10.1016/0005-2736(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket S., Kashket E. R. Dissipation of the proton motive force in oral streptococci by fluoride. Infect Immun. 1985 Apr;48(1):19–22. doi: 10.1128/iai.48.1.19-22.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luoma H., Luoma A. R., Seppä L. Exchange of fluoride between bovine enamel and the surface-related cells of the oral bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(5):343–348. doi: 10.1016/0003-9969(84)90157-2. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Inhibition of streptococcal adenosine triphosphatase by fluoride. J Dent Res. 1977 Jun;56(6):704–704. doi: 10.1177/00220345770560063301. [DOI] [PubMed] [Google Scholar]
- Ophaug R. H., Jenkins G. N., Singer L., Krebsbach P. H. Acid diffusion analysis of different forms of fluoride in human dental plaque. Arch Oral Biol. 1987;32(7):459–461. doi: 10.1016/s0003-9969(87)80004-3. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Protoplast and cytoplasmic membrane preparations from Streptococcus sanguis and Streptococcus mutans. J Gen Microbiol. 1983 Oct;129(10):3271–3279. doi: 10.1099/00221287-129-10-3271. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Downie J. A., Cox G. B., Gibson F., Langman L., Fayle D. R. The uncA gene codes for the alpha-subunit of the adenosine triphosphatase of Escherichia coli. Electrophoretic analysis of uncA mutant strains. Biochem J. 1979 Apr 15;180(1):103–109. doi: 10.1042/bj1800103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S. V., Marquis R. E. Membrane-associated and solubilized ATPases of Streptococcus mutans and Streptococcus sanguis. J Dent Res. 1987 Jun;66(6):1095–1098. doi: 10.1177/00220345870660060201. [DOI] [PubMed] [Google Scholar]
- Tatevossian A. Distribution and kinetics of fluoride ions in the free aqueous and residual phases of human dental plaque. Arch Oral Biol. 1978;23(10):893–898. doi: 10.1016/0003-9969(78)90293-5. [DOI] [PubMed] [Google Scholar]
- Thibodeau E. A., Bowen W. H., Marquis R. E. pH-dependent fluoride inhibition of peroxidase activity. J Dent Res. 1985 Oct;64(10):1211–1213. doi: 10.1177/00220345850640100601. [DOI] [PubMed] [Google Scholar]


