Abstract
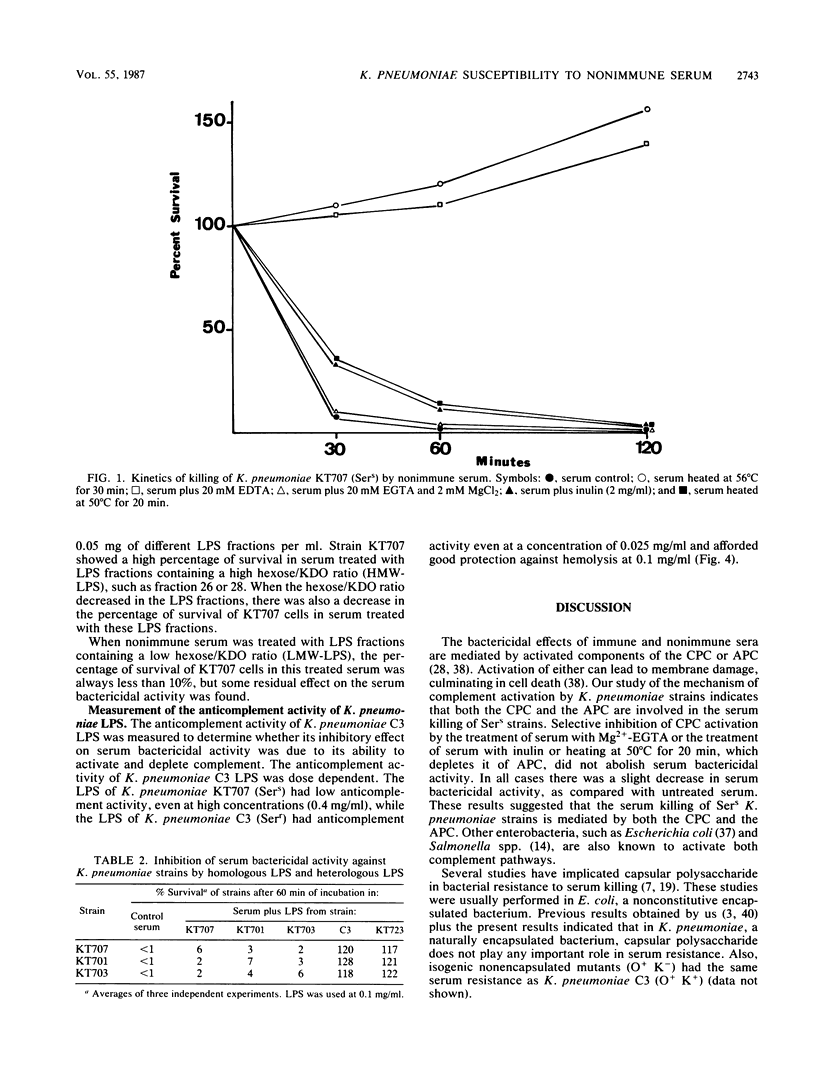
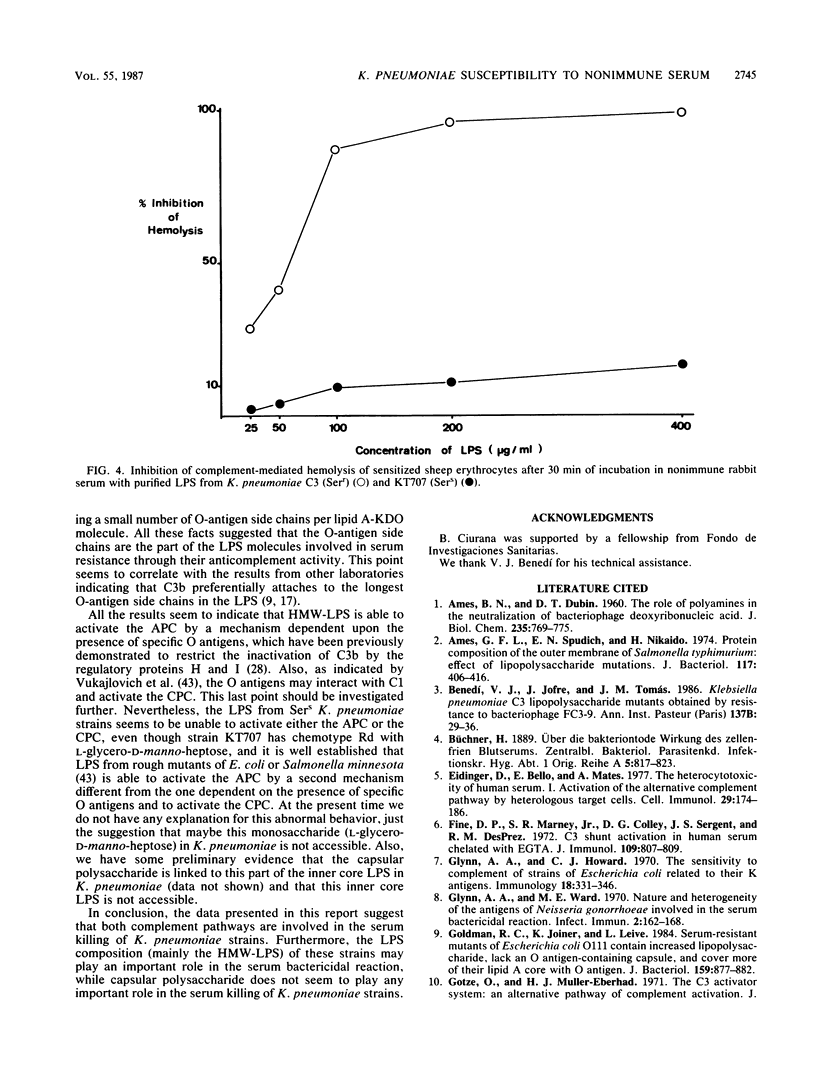
The role of lipopolysaccharide (LPS) in the susceptibility of Klebsiella pneumoniae to serum and the mechanism of complement activation by serum-susceptible (SerS) strains were investigated. The classical and alternative complement pathways are involved in serum killing of susceptible K. pneumoniae strains. The LPS composition seems to play a very important role in the serum bactericidal reaction, while capsular polysaccharide from this bacterium does not play any role. High-molecular-weight LPS from serum-resistant (Serr) K. pneumoniae strains was able to inhibit completely the serum bactericidal activity. LPS from SerS K. pneumoniae strains was not able to inhibit completely the serum bactericidal activity; low-molecular-weight LPS from Serr K. pneumoniae strains could not either. All these findings suggested that LPS composition, especially the O-antigen polysaccharide chains, contributes to the susceptibility of K. pneumoniae strains to complement-mediated serum bactericidal activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedí V. J., Jofre J., Tomás J. M. Klebsiella pneumoniae C3 lipopolysaccharide mutants obtained by resistance to bacteriophage FC3-9. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):29–36. doi: 10.1016/s0769-2609(86)80091-6. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Bello E., Mates A. The heterocytotoxicity of human serum. I. Activation of the alternative complement pathway by heterologous target cells. Cell Immunol. 1977 Mar 1;29(1):174–186. doi: 10.1016/0008-8749(77)90286-6. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Grossman N., Leive L. Salmonellae activate complement differentially via the alternative pathway depending on the structure of their lipopolysaccharide O-antigen. J Immunol. 1983 Apr;130(4):1867–1870. [PubMed] [Google Scholar]
- MacIntyre S., Lucken R., Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986 Apr;52(1):76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Nelson B. W., Roantree R. J. Analyses of lipopolysaccharides extracted from penicillin-resistant, serum-sensitive salmonella mutants. J Gen Microbiol. 1967 Aug;48(2):179–188. doi: 10.1099/00221287-48-2-179. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K. Activation of complement via the alternative pathway. Fed Proc. 1983 Jan;42(1):139–143. [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROANTREE R. J., PAPPAS N. C. The survival of strains of enteric bacilli in the blood stream as related to their sensitivity to the bactericidal effect of serum. J Clin Invest. 1960 Jan;39:82–88. doi: 10.1172/JCI104031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab G. E., Reeves P. R. Comparison of the bactericidal activity of different vertebrate sera. J Bacteriol. 1966 Jan;91(1):106–112. doi: 10.1128/jb.91.1.106-112.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975 Jul;89(1):57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Parton R. A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol. 1977 May;10(2):225–232. doi: 10.1099/00222615-10-2-225. [DOI] [PubMed] [Google Scholar]
- Tomás J. M., Benedí V. J., Ciurana B., Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986 Oct;54(1):85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vukajlovich S. W. Antibody-independent activation of the classical pathway of human serum complement by lipid A is restricted to re-chemotype lipopolysaccharide and purified lipid A. Infect Immun. 1986 Sep;53(3):480–485. doi: 10.1128/iai.53.3.480-485.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukajlovich S. W., Hoffman J., Morrison D. C. Activation of human serum complement by bacterial lipopolysaccharides: structural requirements for antibody independent activation of the classical and alternative pathways. Mol Immunol. 1987 Apr;24(4):319–331. doi: 10.1016/0161-5890(87)90173-8. [DOI] [PubMed] [Google Scholar]